Poly-ADP-ribose polymerase (PARP) inhibitors have added valuable options to the therapeutic landscape in advanced ovarian cancer and have already found their way into ASCO and NCCN guidelines [1–3]. With the addition of another PARP inhibitor to the list of specialties, more patients can now benefit from first-line maintenance therapy [4].
Since October 2021, therapy costs for first-line maintenance therapy of advanced ovarian cancer with the PARP inhibitor niraparib (Zejula®) are covered by health insurance* [4, 5]. The indication expansion is based on positive data from the double-blind Phase III PRIMA study, in which 733 patients with advanced ovarian cancer were randomized to treatment with niraparib or placebo after response to platinum-based chemotherapy. Here, niraparib more than doubled median progression-free survival (PFS) in patients with homologous recombination deficiency (HRD) and reduced the risk of progression or death by 57%. Patients with and without BRCA mutation benefited from this treatment(Table) [6].
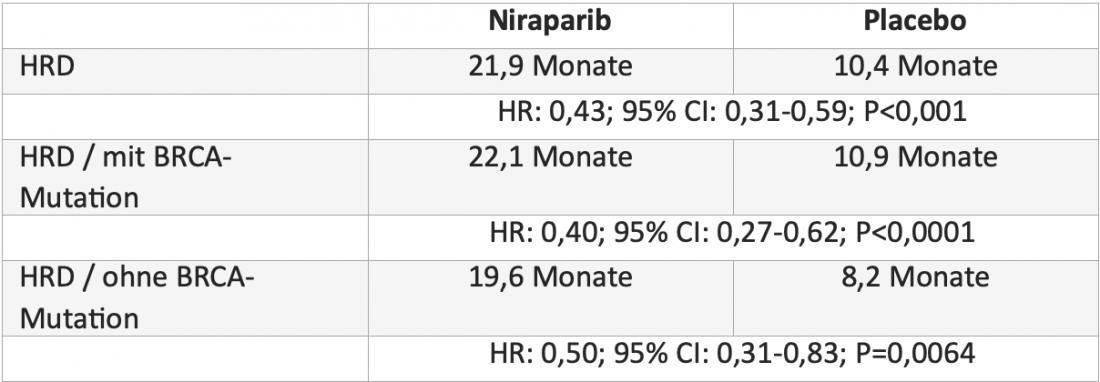 Table: Median progression-free survival with niraparib versus placebo in patients with homologous recombination deficiency (HRD) in the randomized, double-blind phase III PRIMA trial [6].
Table: Median progression-free survival with niraparib versus placebo in patients with homologous recombination deficiency (HRD) in the randomized, double-blind phase III PRIMA trial [6].
Established compatibility and ease of use [5, 7].
Side effects of niraparib were mostly reduced by interruption of therapy or dose reduction. In this regard, individualized starting dosing based on body weight and platelet count helps to reduce side effects without reducing efficacy [5, 8]. Health-related quality of life is also not affected by niraparib [6]. With once-daily oral administration, which can be taken at any time of day regardless of meals, treatment is also convenient and uncomplicated for patients [5].
Niraparib recommended by the guidelines [3].
According to the current ASCO guidelines on the use of PARP inhibitors, all patients with newly diagnosed stage III to IV high-grade serous epithelial ovarian cancer who have had a complete or partial response to first-line platinum-based chemotherapy should be offered maintenance therapy with niraparib regardless of BRCA status [3]. Niraparib treatment is also recommended in the NCCN guidelines regardless of BRCA status [2].
Conclusion
Niraparib is the only once-daily oral PARP inhibitor monotherapy approved for first-line maintenance treatment of patients with advanced ovarian cancer and HRD, regardless of BRCA status [5, 9]. With its established safety profile and practical manageability, the PARP inhibitor offers a patient-friendly therapeutic option with proven efficacy [5, 6].
Anti-PD-1 antibody dostarlimab now approved as a treatment option for recurrent or advanced endometrial cancer (EC) [10]Dostarlimab (Jemperli) has been approved in Switzerland since February 2022 as the first immunotherapy for the treatment of patients with relapsed or advanced EC with defective DNA mismatch repair (dMMR)/high microsatellite instability (MSI-H) that was progressive during or after platinum-based therapy [10,11]. The approval is based on data from the multicenter, single-arm Phase I GARNET study, in which dostarlimab resulted in a durable and clinically meaningful response (objective response rate: 42.3%) in the total of 104 patients with dMMR/ MSI-H studied. The side effect profile was well manageable. The most common third- or higher-degree adverse events were anemia (2.9%), colitis (1.9%), and diarrhea (1.9%) [12]. |
*with limitatio
Literature:
Responsible for content and financed by GlaxoSmithKline AG, Talstr. 3-5, 3053 Münchenbuchsee.
Trademarks are property of their respective owners. © 2022 GSK group of companies or its licensor.
Brief technical information Zejula®
Short subject information Jemperli