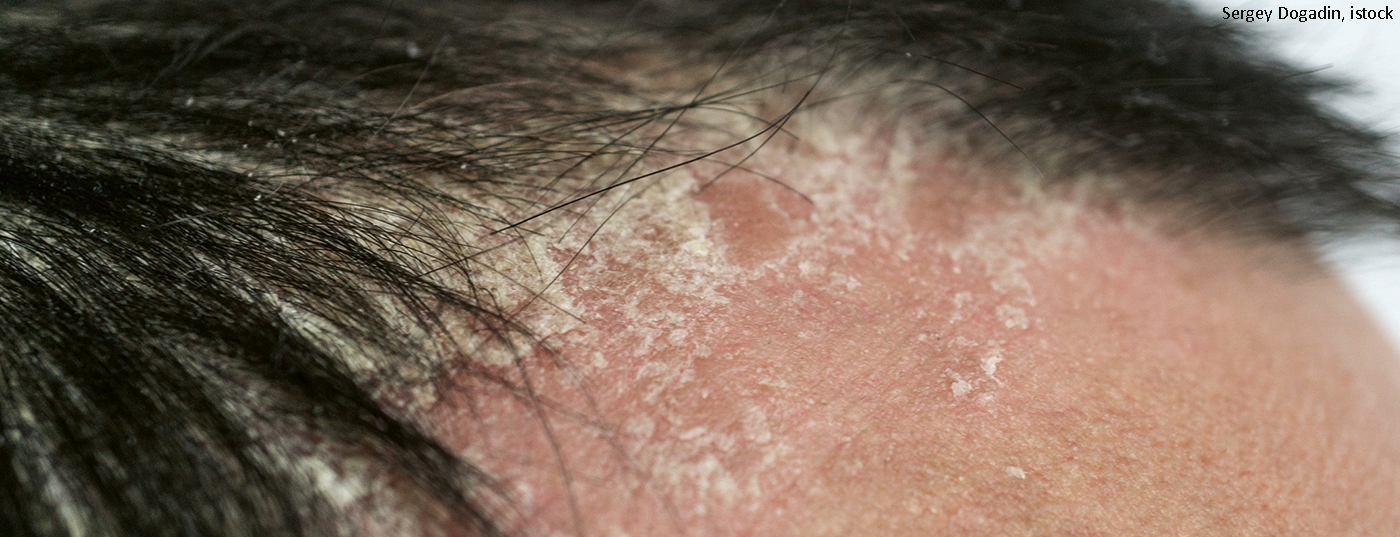
Many psoriasis sufferers experience cutaneous symptoms at highly visible, pruritic localization sites such as the scalp. In a Phase IIIb study, the PDE-4 inhibitor proved to be an effective and safe treatment option for the treatment of moderate to severe psoriasis symptoms on the scalp.
Psoriasis capitis has a significant negative impact on the daily life and quality of life of those affected and often effective treatment proves difficult [1–3]. Topical preparations are used as first-line therapy, but do not always lead to the desired therapeutic success in patients with moderate to severe symptoms [1]. For this patient population, there is a need for well-performing, well-tolerated systemic treatment alternatives. The phosphodiesterase-4 inhibitor (PDE-4 inhibitor) apremilast had been shown to be an effective treatment option in the phase III ESTEEM 1 and 2* trials in patients with moderate to severe plaque psoriasis, including subjects with scalp involvement [4]. Apremilast also performed well in the comparative LIBERATE study, a phase IIIb trial in plaque psoriasis. In another clinical study, the focus was now entirely on the question of the effects with regard to psoriasis symptoms in the area of the scalp.
STYLE study: primary and secondary endpoints met
This placebo-controlled randomized phase IIIb trial evaluated the efficacy and safety of apremilast in adults with moderate to severe psoriasis capitis [6]. This multicenter study involved 41 clinical therapy sites in Canada and the United States, and the study period was from May 2017 to January 2019. 303 patients were randomized in a 2:1 ratio to treatment and placebo arms. In the verum condition, patients received apremilast 30 mg twice daily for a period of 16 weeks. Patients were instructed not to use other treatments for scalp psoriasis during the study period to avoid confounding of treatment effects. An inclusion criterion for the study was that at least one unsuccessful therapy attempt with topical psoriasis treatment had occurred in the past. The primary endpoint was defined as the proportion of patients who achieved a rating of 0 (lesion-free) or 1 (nearly lesion-free) on the scalp therapy scoring system** 16 weeks after baseline and a reduction of at least 2 points. Secondary endpoints included an improvement of at least 4 points regarding itching on the whole body and in the scalp area◊ and an improvement in quality of life (“Dermatology Life Quality Index”) after 16 weeks. In the apremilast condition, significantly more patients achieved a lesion-free or nearly lesion-free scalp (43.3% vs 13.7%) and a reduction in itch in the scalp area (47.1% vs 21.1%), as well as on the whole body (45.5% vs 22.5%) after 16 weeks. Regarding quality of life, a significantly higher proportion of subjects achieved improvement compared to placebo (-6.7 vs -3.8; p<0.0001). No new safety signals emerged; the most common adverse events reported were diarrhea (30.5%) and nausea (21.5%).
* “Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis.”
** “Scalp Physician Global Assessment response”
◊ “Scalp Itch Numeric Rating Scales”
Summary
- Psoriasis in the scalp area can be a significant burden for sufferers, as it is a highly visible site of localization and is often associated with severe itching [1,7].
- The STYLE study is the first prospective, randomized placebo-controlled Phase IIIb study to evaluate the efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp.
- Treatment with apremilast resulted in a significantly higher proportion of patients with lesion-free or nearly lesion-free scalp compared with placebo. The PDE-4 inhibitor also performed significantly better than placebo in terms of scalp and whole body itch reduction and Dermatology Life Quality Index scores. The safety profile of apremilast was consistent with that of previous studies, and no new safety signals emerged [8,9].
Literature:
- Blakely K, Gooderham M: Management of scalp psoriasis: current perspectives. Psoriasis (Auckl) 2016; 6: 33-40.
- Heydendael VM, et al: The burden of psoriasis is not determined by disease severity only. J Investig Dermatol Symp Proc 2004; 9: 131-135.
- Kim TW, et al: Clinical characteristics of pruritus in patients with scalp psoriasis and their relation with intraepidermal nerve fiber density. Ann Dermatol 2014; 26: 727-732.
- Rich P, et al: Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with difficult-to-treat nail and scalp psoriasis: results of 2 phase III randomized, controlled trials (ESTEEM 1 and ESTEEM 2). JAAD 2016; 74: 134-142
- Reich K, et al: The efficacy and safety of apremilast, etanercept, and placebo, in patients with moderate to severe plaque psoriasis: 52-week results from a phase 3b, randomized, placebo-controlled trial (LIBERATE). JEADV 2017; 31: 507-517
- Van Voorhees AS, et al: Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. JAAD 2020; 83(1): 96-103.
- Kim J, Krueger JG: The immunopathogenesis of psoriasis. Dermatol Clin 2015; 33: 13-23.
- Papp K, et al: Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]). J Am Acad Dermatol 2015; 73: 37-49.
- Paul C, et al: Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol 2015; 173: 1387-1399.
DERMATOLOGY PRACTICE 2020; 30(4): 23