Among other things, new findings on the therapy of diffuse large B-cell lymphoma were presented. The phase III FLYER trial compared standard therapy (6xR-CHOP) with treatment reduced by two cycles of chemotherapy. Another study has succeeded in developing a new molecular genetic method as the basis for a personalized therapy strategy.
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin’s lymphoma worldwide and belongs to the so-called aggressive non-Hodgkin’s lymphomas. The standard therapy to date has been immunochemotherapy 6xR-CHOP. 6xR-CHOP combines monoclonal antibodies (R) with cytostatic drugs (CHOP) in six cycles [1]. The empirical basis for this treatment regimen comes from the MINT trial [3]. In patients with an age-adjusted prognostic index (aaIPI) score of 0 and no tumor mass, six cycles of therapy resulted in a 3-year EFS of 89% and a 3-year PFS of 95%, and a 3-year OS of 98% [3].
In the FLYER trial, it was shown that two cycles of chemotherapy can be omitted without having a negative impact on disease progression. A shorter duration of chemotherapy means that patients can return to a normal daily routine and to work more quickly, as the study’s first author, Dr. Viola Poeschel, Saarland University Medical School in Homburg/Saar (Germany), explains [1].
FLYER multicenter study: reduction of chemotherapy without negative effects
The FLYER international prospective multicenter study (n=588) investigated the effect of reducing cytostatic treatment cycles of standard therapy on disease-relevant parameters. For this purpose, 6xR-CHOP (n=295) was compared with a regimen of 4xR-CHOP+2xR (n=293) over an eleven-year period (2005-2016) in a randomized, balanced noninferiority design. The 18- to 60-year-old patients had stage I or II DLBCL without tumor mass and were randomly assigned to one of the two study arms (six vs. four cycles of CHOP and six doses of rituximab each). Each of the R-CHOP cycles occurred at an interval of 21 days. The primary endpoint was non-inferiority in progression-free survival (PFS). Secondary endpoints were various disease-related parameters. 4xR-CHOP+2xR was shown to be non-inferior to standard 6xR-CHOP; cancer cell clearance and relapse prevention were comparably effective with four cycles of chemotherapy as with six cycles. In terms of tolerability, the reduction of chemotherapy by two cycles had a favorable effect [1].
The 3-year PFS rate (primary endpoint) after a median follow-up of 66 months was 94% (95% CI, 91-97) in the 6xR-CHOP condition (n=295) and 96% (95% CI, 94-99) in the 4xR-CHOP+2xR condition (n=293). Also, with regard to 3-year OS rate after a median follow-up of 67 months, the results differed only slightly 98% (95% CI, 96-99) at six cycles versus 99% (95% CI, 98-100) at four cycles [1].
Regarding tolerability, the results were as follows: Compared with the 6xR-CHOP condition (1295 AEs), the total number of AEs in the 4xR-CHOP+2xR condition (835 AEs) was about one-third lower [1]. The total number of grade 3 and grade 4 non-hematologic adverse events (AEs) was higher in the 6xR-CHOP treatment arm (70 AEs) compared with the 4-cycle arm (46 AEs) [1,4]. Regarding hematologic AEs, the number of affected individuals was higher in the 6 R-CHOP condition compared with 4xR-CHOP+2xR: grade 3 or 4 leukopenia (110 vs. 80), anemia (8 vs. 2) and thrombocytopenia (7 vs. 5). Also, in the 6xR-CHOP condition, more patients were affected by grade 3 and 4 paresthesia (14 vs. 12), nausea (12 vs. 6), infection (23 vs. 20), vomiting (7 vs. 1), and mucositis (3 vs. 1). In order to be able to make a statement as to whether a reduction in the number of R-CHOP cycles has an impact on the negative long-term effects of chemotherapy, monitoring over a further period of five years is required [1].
Personalization of treatment: new molecular genetic analysis
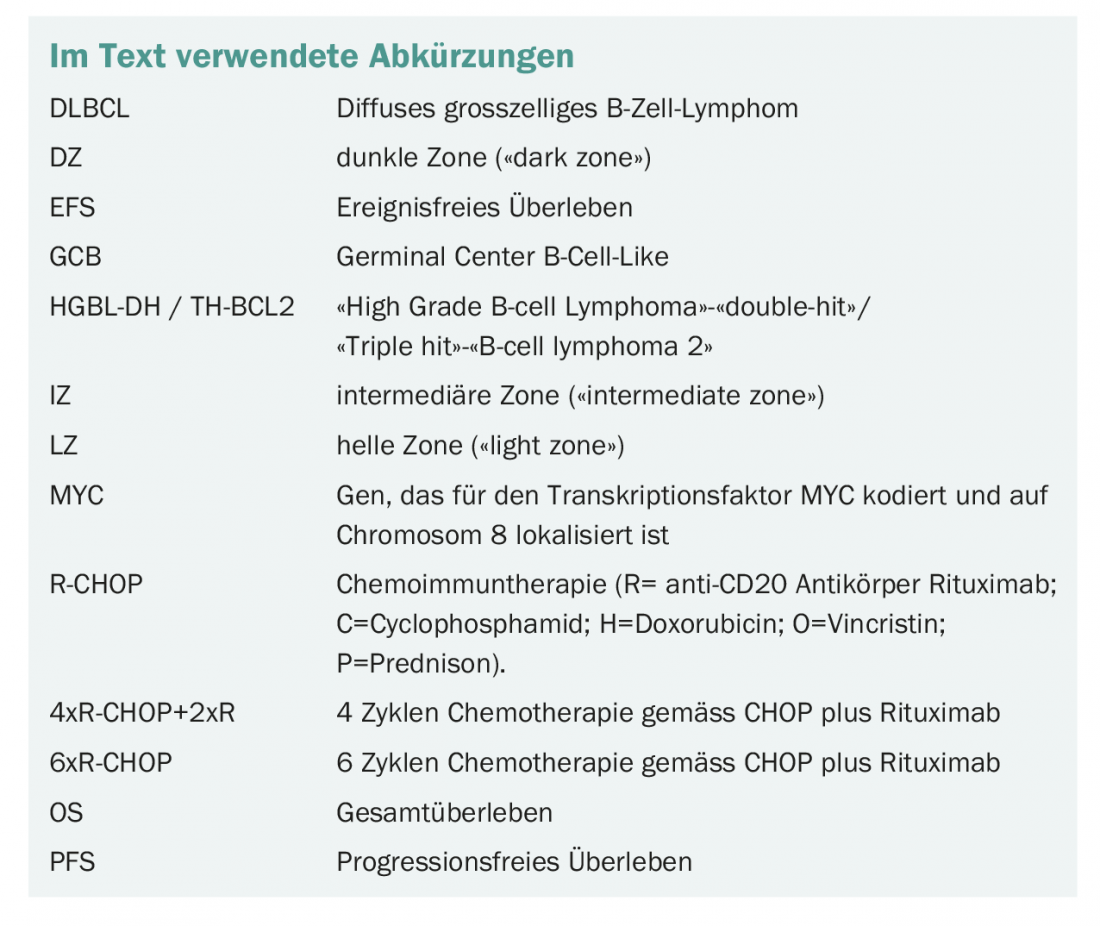
Diffuse large B-cell lymphomas can be subdivided according to morphological criteria (centroblastic, immunoblastic, anaplastic), gene expression (“germinal-center B-cell (GCB)-like”, “activated B-cell (ABC)-like”), by immunohistochemical features (in particular CD5, CD30, MYC, BCL2, BCL6, GCB-like, non-GCB-like), and by genetic abnormalities (in particular translocation of MYC, BCL2, and/or BCL6). In the most recent edition of the WHO classification, the definition of lymphoma types was revised to include findings from new molecular analysis methods such as next generation sequencing (Table 1) [5].
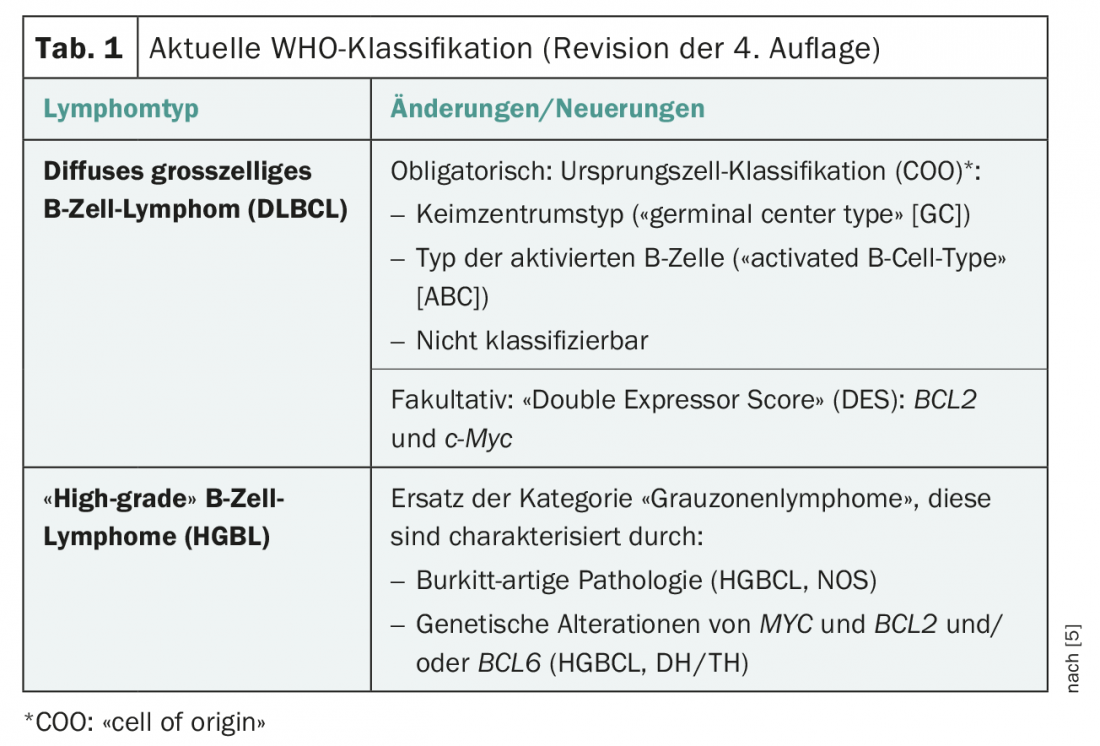
Identifying molecular subgroups is a potential basis for personalizing treatment. A study presented at ASH and published concurrently in the Journal of Oncology addresses this approach [2]. Here, within the “germinal-center B-cell (GCB)-like” DLBCL (GCB-DLBCL) variant, a subgroup was identified that differs clinically and biologically in a signature of HGBL-DH/TH-BCL2 gene expression. The background of this study was the finding that high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 (HGBL-DH/TH) respond poorly to standard therapy (chemoimmunotherapy). To analyze the molecular characteristics of HGBL-DH/TH with BCL2 (HGBL-DH/TH-BCL2), as well as the morphology of DLBCL, a 104-gene double-hit signature (DHITsig) was developed as a distinguishing feature between HGBL-DH/TH-BCL2 from other GCB-DLBCLs.
DHITsig identifies tumors that differ in biological characteristics that may be relevant to progression and therapy. DHITsig-pos tumors are characterized by a COO of the intermediate/dark zone of the germinal center (GC) type and have significantly lower expression of light zone genes compared to DHITsig-neg tumors (p<0.001) (Fig. 1). DHITsig-positive patients were found to have a worse outcome after R-CHOP immunochemotherapy compared to DHITsig-negative patients (progression-free survival over 5 years, 57% and 81%, respectively; p<0.001), independent of HGBL-DH/TH-BCL2 status.
Expression of MYC in normal germinal center cells is restricted to cells located within the LZ for reentry into the DZ. The authors of the study suggest that these recycling cells represent the physiological counterpart of DHITsig-pos tumor cells. In addition to a high incidence of mutations within chromatin-modifying genes, they exhibit a high incidence of low MHC-I and MHC-II expression and a high expression level of genes associated with oxidative phosphorylation.
According to the authors, these biological features may eventually provide a basis for the development of targeted active compounds beyond the current focus on BCL2 inducers. Possible therapeutic approaches that can be derived from this relate to cell differentiation (“enhancers of zeste homolog 2 inhibitors”), reduction of immune evasion (“histone deacetylase inhibitors”), increase of T-cell activation (anti-CD20 antibodies of the newer generation), as well as oxidative phosphorylation and the proteasome. The high expression levels of certain genes also indicate that specific inhibitors may be beneficial (e.g., inhibitors of arachidonate 5-lipoxygenase).
The subgroup analysis by gene expression signature DHITsig developed in this study can be used for studies of biopsy samples in a clinical context.
Source: 60th ASH Meeting, December 1-4, 2018, San Diego (USA).
Literature:
- Poeschel V, et al.: Excellent outcome of young patients (18-60 years) with favorable-prognosis diffuse large B-cell lymphoma (DLBCL) treated with 4 cycles CHOP plus 6 applications of rituximab: results of the 592 patients of the FLYER trial of the Dshnhl/GLA. Oral and Poster Abstracts, Abstract No. 781: Session: 626. Presented at: ASH Annual Meeting and Exposition; December 4-8, 2018; San Diego, California. Abstract 781, https://ash.confex.com/ash/2018/webprogram/Paper112403.html
- Ennishi D, et al: Double-hit Gene Expression Signature Defines a Distinct Subgroup of Germinal Center B-Cell-Like Diffuse Large B-Cell Lymphoma. Journal of Clinical Oncology 2018; Dec 3:JCO1801583. doi: 10.1200/JCO.18.01583. [Epub ahead of print], Presented at the 60th American Society of Hematology Annual Conference, San Diego, CA, December 1-4, 2018.
- Pfreundschuh M, Truemper L, Osterborg A, et al: CHOP-like chemotherapy plus rituximab compared with CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large B-cell lymphoma: a randomized controlled trial by the Mabthera International Trial (MInT) Group. Lancet Oncology 2006; 7(5): 379-391.
- NIH: National Cancer Institute. Division of Cancer Treatment and Diagnosis. Common Terminology Criteria for Adverse Events, https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40, last accessed Dec. 19, 2018.
- Menter T, Dirnhofer S: WHO 2017: progress, regression or sideways step? Switzerland Med Forum 2018; 18(03): 52-54 DOI: https://doi.org/10.4414/smf.2018.03150.
InFo ONCOLOGY & HEMATOLOGY 2019; 7(1): 30-31.