Over time, fluctuations in efficacy may occur in Parkinson’s therapy with L-dopa. Then, the treatment of L-dopa induced dyskinesias and motor fluctuations represents the most important task in clinical practice. Additional blockade of COMT may result in a prolongation of the L-dopa half-life and an increase in bioavailability.
More than 50 years after its introduction into routine clinical practice, L-dopa remains the best-performing substance for controlling motor symptoms of Parkinson’s disease and continues to be one of the best-tolerated Parkinson’s drugs [1,2]. However, chronic long-term therapy with L-dopa is associated with the development of motor complications in the form of fluctuations in effect and drug-induced dyskinesias in a proportion of treated patients. The frequency of these complications associated with L-dopa therapy varies depending on the duration of the disease and therapy, the L-dopa dose selected, and the age of the patient. In controlled therapy studies, between and 20 and 30% of patients developed motor complications in the first few years of therapy, and this rate increases in most long-term series to at least 50% after more than five years of treatment [3,4]. Surveys of patients regularly show that fluctuations in effect with partly unpredictable episodes of recurrent Parkinson’s symptoms are more stressful than drug-induced agitation – as long as the latter does not reach a disabling level [5]. The treatment of -L-dopa-induced dyskinesias and motor fluctuations continues to be one of the most important tasks in both clinical practice and therapeutic research in Parkinson’s disease.
Motor fluctuations under L-dopa – clinical spectrum and underlying mechanisms.
The most common and also often initial presentation of effect fluctuations with L-dopa is the recurrence of parkinsonian symptoms toward the end of dosing intervals of an ongoing treatment with repeated intakes four or more hours apart and is referred to as the “wearing-off” phenomenon. The decreases in effect are perceived by patients as a return or increase in classic motor signs of Parkinson’s such as tremor, muscle stiffness, and immobility, but are also usually coupled with non-motor symptoms such as feelings of listlessness, thought blocks, depressiveness, anxiety, or pain [6]. This combination of disturbed mobility with additional disturbances of well-being make off-phases particularly unpleasant for patients [7]. Without specific questioning about the temporal association of complained symptoms with the times of taking L-dopa den, wearing-off phenomena may escape correct attribution, as studies using wearing-off questionnaires have shown [8]. Other manifestation forms of impact fluctuations are summarized in Figure 1.
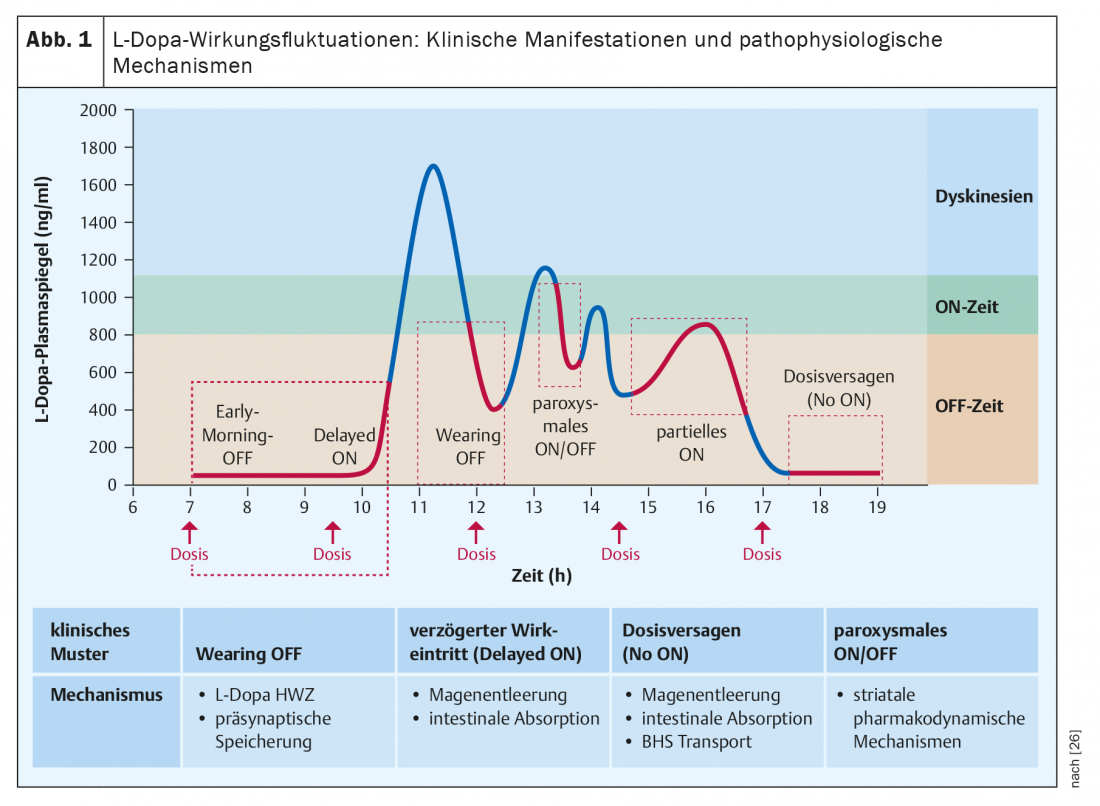
Pathophysiologically, the short half-life (about 90 minutes) of L-dopa is mainly responsible for the wearing-off fluctuations, leading to regular alternation of blood level maxima and minima during administration of several single doses distributed throughout the day. With decreasing neuronal storage capacity of dopamine formed from exogenous L-dopa during the course of the disease, these peripheral oscillations also lead to fluctuations in synaptic dopamine availability. Phenomena such as delayed onset of action of an oral dose or even complete loss of action of a single dose are usually due to impaired gastrointestinal absorption (delayed gastric emptying, competition of intestinal L-dopa absorption with dietary amino acids), whereas sudden and unexpected onset of action, seemingly unrelated to time of intake, are based on central pharmacodynamic alteration [9]. (Fig. 1).
The most important risk factors for the development of motor fluctuations are the duration of the disease, the level of L-dopa dose, and the age of the patients [10]. While the increasing risk of complications with disease duration is explained by the progressive loss of nigrostriatal projection neurons and thus the storage capacity of dopamine formed from exogenous L-dopa, the age effect (greater risk in younger patients) has not been definitively elucidated. Similar is the effect of L-dopa dose (higher dose per body weight corresponds to higher risk).
Drug treatment options for motor fluctuations.
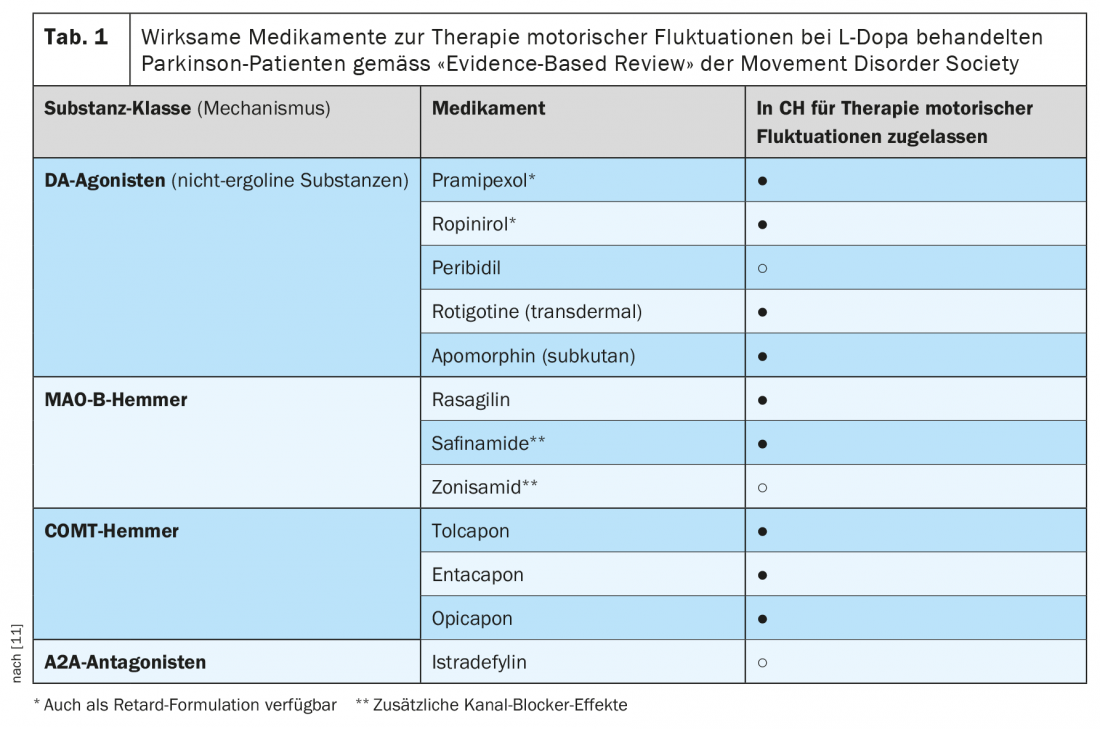
Basically, three dopaminergic drug classes are available for the oral pharmacotherapy of patients with L-dopa effect fluctuations, as well as adenosine A2 receptor antagonists for adjuvant therapy in the USA and Japan [11] (Table 1). Dopamine agonists (standard oral preparations, sustained-release preparations, and a transdermal formulation) are able to compensate for fluctuations in the effect of L-dopa due to their significantly longer half-life compared with L-dopa (or continuous delivery in the case of the patch formulation). The effect size of these adjunctive therapies in clinical trials was in the range of a reduction of two to three hours in cumulative daily off-time detectable with patient diaries. Inhibitors of monoamine oxidase-B (MAO-B inhibitors) prolong L-dopa action via blockade of the central degradation pathway of synaptically released dopamine and have been shown in clinical trials to reduce daily off-time to the extent of approximately one hour. COMT inhibitors prolong L-dopa action by altering peripheral pharmacokinetics and have effect sizes ranging from just under one hour to just under two hours off-time reduction. Istradefyline, an inhibitor of adenosine A2 receptors, is also approved in Japan and the United States for the treatment of action fluctuation The principle of action of A2 A antagonists is to enhance dopaminergic signal transduction in the so-called indirect projection pathway from the striatum into the globus pallidus. Recent studies of the treatment of L-dopa-induced dyskinesia with a sustained-release formulation of amantadine, now approved in the United States, have also demonstrated an effect of this nondopaminergic drug on effect fluctuations [12].
In addition to the above-mentioned therapeutic measures, individual off-phases can also be treated with so-called “on-demand” therapies such as subcutaneous apomorphine injections, and more recently in the USA also sublingual apomorphine administration or L-dopa inhalation. For patients with refractory effect fluctuations who do not respond satisfactorily to all of these measures, both intestinal L-dopa infusions and subcutaneous apomorphine continuous infusions are available [13].
Role of COMT inhibitors in the treatment of effect fluctuations.
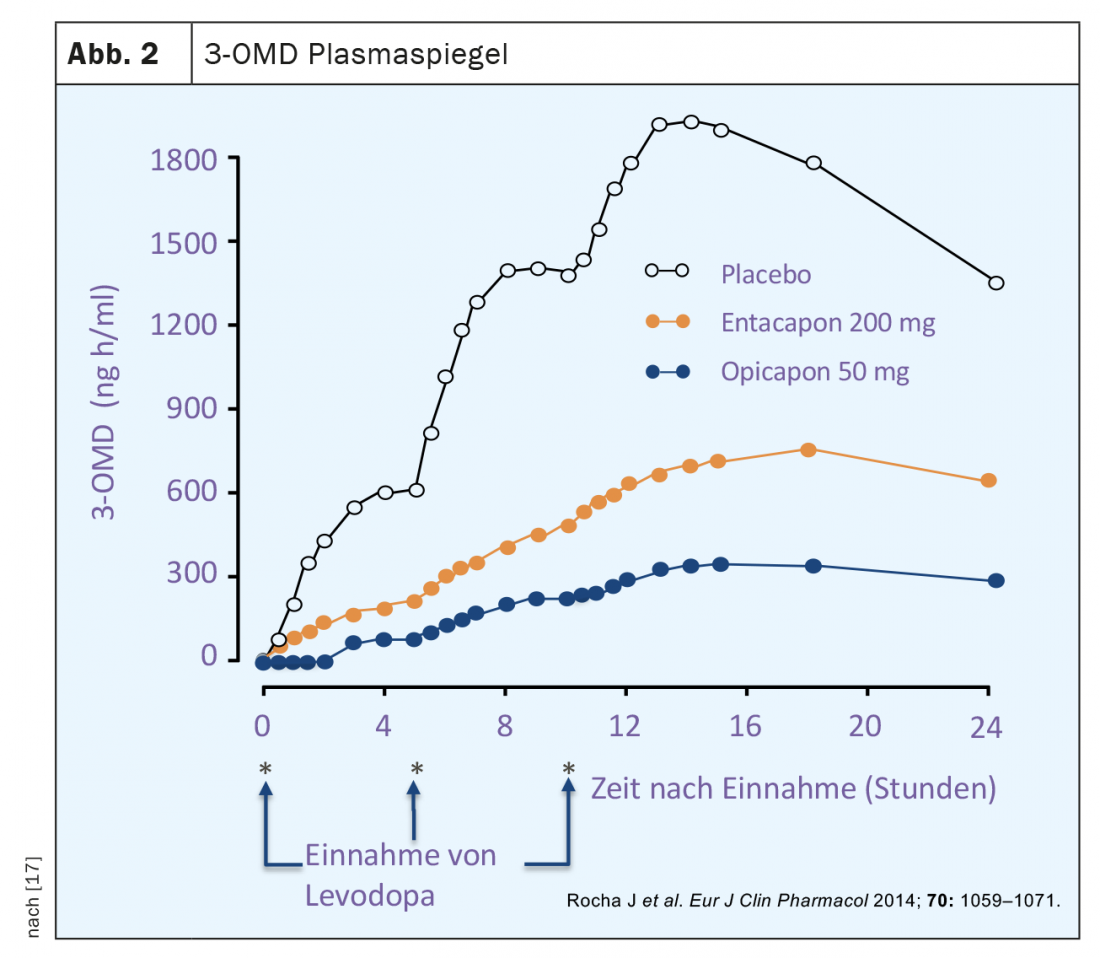
Classical L-dopa therapy takes the form of combination preparations of L-dopa with a peripherally acting inhibitor of aromatic amino acid decarboxylase (AADC). In this way, dopamine is prevented from being generated from orally administered L-dopa already in the periphery, especially in the liver. Before the introduction of such inhibitors, L-dopa-only therapy was associated with a much higher dose requirement and also peripheral dopaminergic side effects, particularly nausea and hypotension. However, despite inhibition of AADC, hepatic metabolism still occurs with classical L-dopa therapy using the otherwise less used degradation pathway via catechol-o-methyl transferase. This metabolic pathway of L-dopa leads to the formation of the major metabolite 3-o-methyl-dopa (3-OMD), whose half-life is significantly longer than that of L-dopa and whose blood levels in L-dopa-treated PD patients are several times higher than those of L-dopa (Fig. 2).
Additional blockade of COMT (double enzyme inhibition of AADC and COMT) leads to a prolongation of the L-dopa half-life as well as to an increase in bioavailability [14]. Historically, tolcapone was the first COMT inhibitor introduced into Parkinson’s therapy, showing very good efficacy with a reduction in daily off-time of up to two hours, but had to be temporarily withdrawn from the market in the EU due to serious hepatotoxicity and is now only used as a second-line drug under close monitoring of liver function. The COMT inhibitor entacapone, approved approximately one year after the initial approval of tolcapone in 1997, is free of such hepatotoxic effects but is weaker in efficacy than tolcapone [15]. Both first-generation COMT inhibitors can produce diarrhea, forcing discontinuation of therapy in 3% to 5% of those treated, and cause urine discoloration due to the coloring effect of the metabolites of these two nitro-catechol compounds excreted in the urine [14].
In 2016, opicapone, a new peripherally acting selective COMT inhibitor for the treatment of effect fluctuations, was approved in the EU and has also been available in Switzerland since 2020.
Pharmacology of opicapone
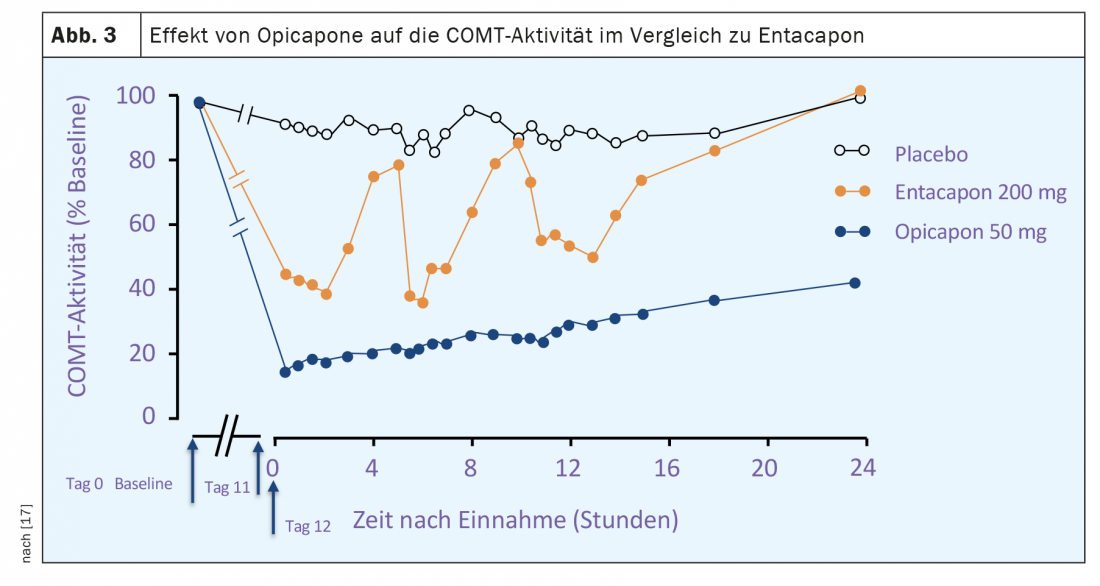
Opicapone is a selective peripherally acting inhibitor of COMT. Compared with entacapone, there is a high binding affinity to the enzyme and a slower dissociation rate of the opicapone of the COMT complex [16]. The latter implies a much longer duration of action compared to entacapone or tolcapone with the possibility of once-daily dosing of opicapone. Studies of COMT activity in erythrocytes from both healthy volunteers and Parkinson’s disease patients showed a maximum reduction of up to 100% and of about 60% 24 hours after a single dose. (Fig. 3). The stronger inhibition of COMT activity by opicapone compared with entacapone was also reflected in a greater reduction in plasma levels of 3 OMD (Fig. 2). The bioavailability (area under the curve, AUC) of a single dose of L-dopa plus decarboxylase inhibitor is increased by up to 35% by opicapone in a dose-dependent manner.Opicapone is mainly metabolized to inactive metabolites in the liver and approximately 70% of excretion occurs via hepatobiliary excretion with feces. In patients with moderate hepatic impairment (Child-Pugh B), there are significant increases in opicapone concentration and bioavailability compared to healthy individuals, so dose adjustments may be necessary. For use in patients with severe hepatic impairment (Child-Pugh C), no data are available and use of opicapone is not recommended here. In Switzerland, the use of opicapone is generally not recommended in cases of liver dysfunction (Child-Pugh A,B,C) or the presence of liver cirrhosis.
Clinical studies on the efficacy of opicapone
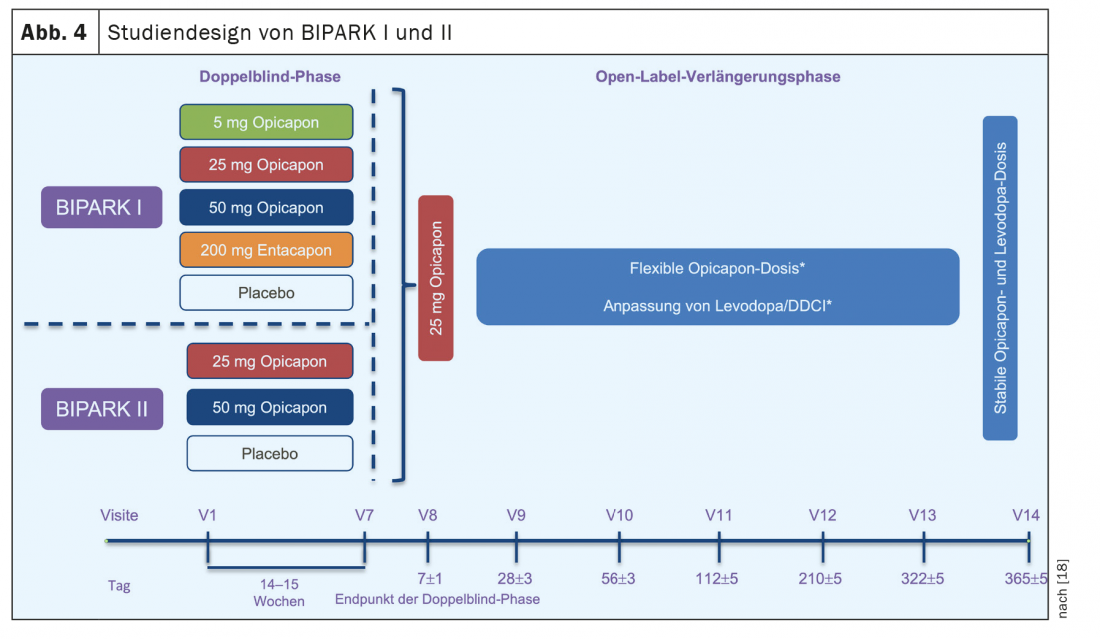
The approval of opicapone for the treatment of motor fluctuations in L-dopa-treated Parkinson’s patients is based on evidence of efficacy from two large double-blind Phase III studies (BIPARK I and II), which included a total of more than 1000 patients. [18] (Fig. 4). In BIPARK I, three doses of opicapone (5 mg, 25 mg, 50 mg) were compared with placebo and entacapone as the active comparator, while in BIPARK II, 25 and 50 mg opicapone were compared with placebo. The median disease duration of the included patients ranged from 7 to 8 years, and at study inclusion, the median daily off time was approximately six hours. The primary endpoint of both studies was the change in daily off-time between study inclusion and the end of the double-blind treatment phase after 15 weeks. Secondary analyses determined the extension of on-time during the same period, changes in percent off-time and on-time, the number of so-called off-time responders with reductions of at least one hour, and on-time responders with extensions of at least one hour. In addition, global assessments of changes in PD by investigators and patients and quality-of-life scales (PDQ-39) were evaluated.
Study results related to the primary endpoint of BIPARK I and BIPARK II are shown in Figure 5. The mean off-time reduction under 50 mg opicapone was just under two hours and was numerically greater than in the treatment arm with entacapone (1.6 hr). However, the comparison between opicapone and entacapone in BIPARK I was designed to demonstrate non-inferiority only; statistically significant superiority of opicapone could not be demonstrated in the chosen design. The placebo effect was similar in both studies (approximately one hour), and the changes in on-time were mirrored by the OFF-time reduction in both studies (1.4 and 1.8 hours, respectively, for 50 mg opicapone compared with 0.8 hours for placebo).

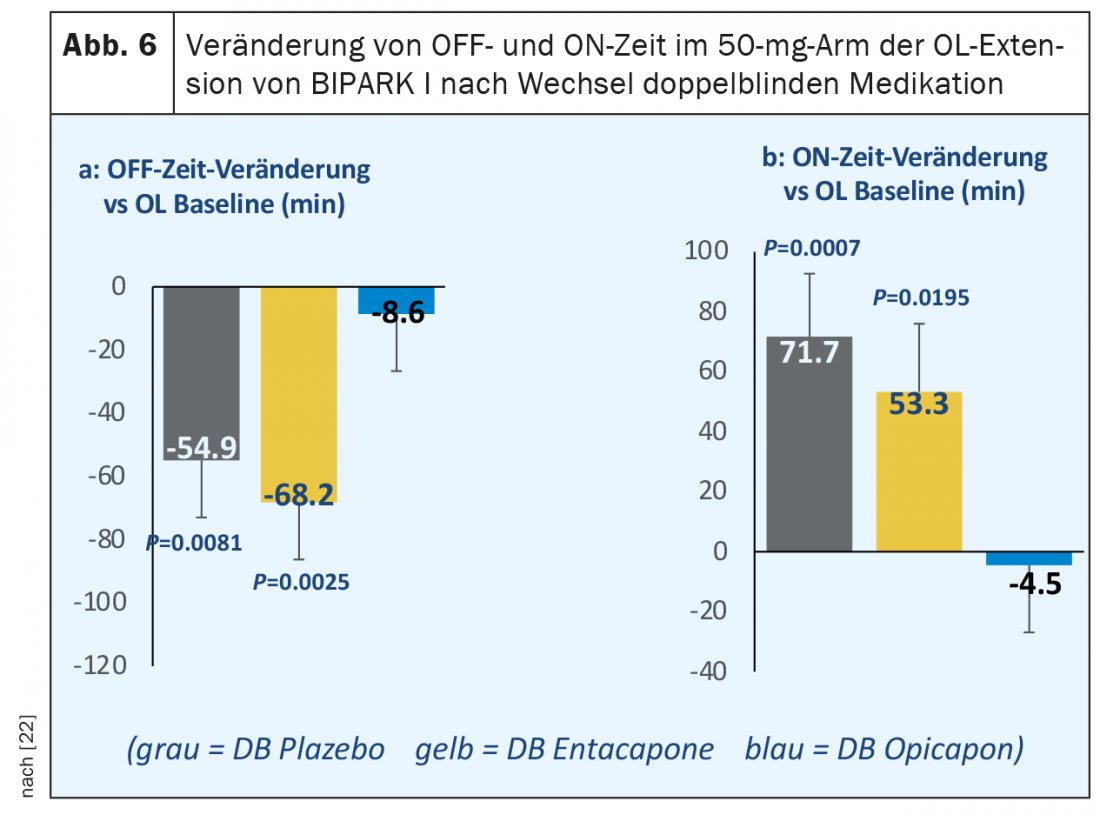
After completion of the three-month double-blind phase of BIPARK I and BIPARK II, patients were offered continuation of open-label treatment with opicapone in a one-year extension phase. Here, all patients initially received 25 mg opicapone once daily with the possibility of dose adjustment during the course. The most significant findings from these extension studies were the persistence of the effect on off-time reduction and on-time prolongation in those patients who had already been treated with opicapone in the double-blind phase. In the BIPARK I extension study, a further statistically significant improvement in absolute daily off-time of 40 minutes was also observed after switching from double-blind entacapone to open-label treatment with 50 mg opicapone [22]. In a post-hoc analysis of this open-label extension study, the effects were even more striking in a subgroup of 122 patients treated with 50 mg opicapone until the end of the study, with an approximately 70-minute reduction in off-time compared with double-blind entacapone treatment and a gain of 53 minutes of on-time without distressing dyskinesias (Fig. 6). Another post-hoc analysis determined the proportion of those patients who showed a reduction in daily off-time or prolongation of daily on-time by at least two hours from baseline to the end of the study (so-called “super-responders”) during the double-blind phase of BIPARK I and BIPARK II under 50 mg opicapone. Of a total of 265 patients in both studies combined, 100 (37%) met this criterion (Antonini et al., poster EAN 2020).
Tolerability of opicapone in clinical studies
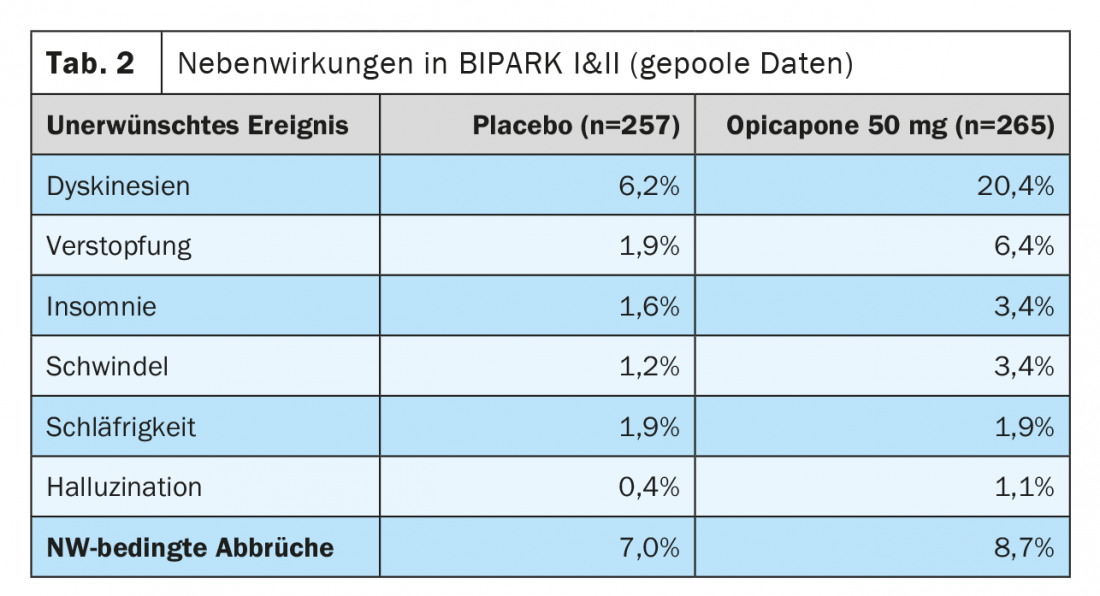
The most common adverse events for opicapone in the studies summarized above were dyskinesia, which occurred in 16% in the 50 mg dosing arm (BIPARK I) and 24% (BIPARK II) were reported compared to 4% and 8% in the placebo arms of the two studies (Table 2). Approximately 6% of patients reported constipation in BIPARK I and BIPARK II taking 50 mg opicapone, while no cases of clinically relevant diarrhea or urine discoloration were reported. Similarly, in BIPARK I and II (including the open-label extension studies), there was no evidence of hepatic toxicity and there were no differences in side-effect-related study discontinuations between opicapone and placebo [18,23,24].
Use of opicapone in clinical practice
The recommended daily dose of opicapone is 50 mg and it should be taken as a single dose one hour before or after a dose of L-dopa. The concomitant use of L-dopa preparations and Opicapone can lead to a significant increase in the rate of absorption of L-dopa, so that from a pragmatic point of view it is recommended to take Opicapone before bedtime, which usually makes it easy to ensure the one-hour interval from the last daily dose. Switching from previously existing treatment with entacapone or tolcapone to opicapone can be done within 24 hours without a ‘washout’ period, as COMT inhibition of either drug lasts less than 24 hours. While some of the limiting side effects of entacapone or tolcapone (diarrhea, urinary discoloration, or hepatotoxicity with tolcapone) have not been observed to date, opicapone may also cause dopaminergic side effects by increasing the bioavailability of L-dopa. Most commonly, this is the induction or exacerbation of drug-induced dyskinesia, which can usually be corrected by reducing the daily dose of L-dopa or concomitant dopaminergic therapies such as MAO-B inhibitors or dopamine agonists. In patients who already have functionally relevant dyskinesias prior to the addition of Opicapone, a reduction of the L-dopa dose by 20-30% concurrent with the start of treatment may be considered. In this case, however, a short-term control (in the consultation or by telephone) should check whether a deterioration in the Parkinson’s status has occurred as a result of the reduction, and further adjustments should be made if necessary. An increased risk of adverse dopaminergic effects after addition of opicapone also exists in patients with clinically relevant cognitive dysfunction, particularly with a history of drug-induced hallucinosis or confusion. Again, short-term monitoring is required, as well as medication adjustments if necessary (dopaminergic dose reduction, addition of quetiapine or clozapine).
A recent observational study of 495 patients in a clinical practice setting showed an overall rate of treatment discontinuation due to side effects of 17% in the first three to six months. Dyskinesias occurred in 11% but were a reason for treatment discontinuation in only 1% [25]. In contrast, the proportion of patients who assigned themselves to one of the three degrees of improvement (little to much better) in the self-assessment scale (‘Patient Global Impression of Change’, P-GIC) after 3 months of treatment with opicapone was 77% (48% “much” or “very much” better).
Summary
The addition of COMT inhibitors is an effective strategy for improving motor fluctuations under ongoing treatment with L-dopa, as demonstrated in numerous high-quality studies [11]. Entacapone and opicapone are among the first-choice drugs in this indication, whereas tolcapone is a 2nd-choice agent because of its hepatotoxicity. Pharmacological COMT inhibition is the only approach in Parkinson’s drug therapy that directly modifies one of the most essential pathogenetic factors for the development of effect fluctuations-the short half-life of L-dopa. This makes the combined administration of L-dopa and COMT inhibitors in patients with these motor complications a rational approach that should be used early. For theoretical reasons, it has even been postulated that a combination with a COMT inhibitor already used at the beginning of L-dopa treatment could lead to the prevention of motor complications – but a single study investigating this so far found negative effects in terms of faster and more frequent dyskinesias over the course of up to four years [10].
To date, COMT inhibitor therapy has been limited by the toxicity of the highly potent tolcapone and the weaker effect of entacapone compared with tolcapone. In addition, there were intestinal intolerance reactions with severe diarrhea and undesirable urine discoloration caused by both substances. With opicapone, a new highly potent representative of this class has become available, which has several advantages: the inhibitory effect on COMT is significantly stronger than that of entacapone, which has also been reflected in numerically larger effects on daily OFF-time reduction in clinical trials compared with entacapone. The duration of COMT inhibition by opicapone is long-lasting, allowing once-daily dosing, and diarrhea or urine discoloration have not been observed in clinical trials. This should spur the use of COMT inhibitors in the management of effect fluctuations, but with the caveat that enhancement of L-dopa effects may also lead to dopaminergic side effects. Foremost among these are dyskinesias, but these can almost always be controlled by dose reduction of L-dopa. L-dopa ‘saving effects’ through addition of potent COMT inhibitors such as opicapone can also be used in practice to avoid increasing L-dopa dosing frequency.
COMT inhibition is a rational approach to optimize L-dopa delivery in PD patients with fluctuations in effect, which is also useful and effective in combination with other drug classes (Table 1) . A prerequisite for timely treatment is the timely clinical diagnosis of wearing-off fluctuations, which can only be achieved by allowing sufficient time for targeted questioning of patients and, if necessary, relatives or caregivers.
Take-Home Messages
- More than half of Parkinson’s patients treated with L-dopa develop motor fluctuations (on-off fluctuations) in the long-term course. Off-phases are particularly unpleasant for sufferers due to the combination of motor and non-motor symptoms, yet ‘wearing-off’ fluctuations are easily overlooked in practice without targeted questioning.
- The short half-life of L-dopa leads to dose-dependent blood level fluctuations and is a central factor in the pathogenesis of effect fluctuations under L-dopa COMT inhibitors interfere with peripheral L-dopa metabolism and lead to prolongations of half-life and bioavailability. They are therefore first-choice drugs for reducing fluctuations in effect under L-dopa.
- Opicapone is a new peripherally acting COMT inhibitor with a long duration of action and the option of once-daily dosing. The inhibitory effect on COMT is greater compared with entacapone, and clinical trials have shown numerically greater reductions in daily OFF time.
- Opicapone has advantages over previous COMT inhibitors in lack of induction of diarrhea or urine discoloration. The most important side effect of opicapone for practice is a possible enhancement of L-dopa-induced dyskinesias, so L-dopa dose reductions may be required.
- The addition of COMT inhibitors is a rational and obvious pharmacological measure to optimize L-dopa pharmacokinetics when effect fluctuations occur.

Literature:
- Djamshidian A, Poewe W: Apomorphine and levodopa in Parkinson’s disease: two revolutionary drugs from the 1950s. Parkinsonism Relat Disord 2016; S9-S12.
- LeWitt PA, Fahn S: Levodopa Therapy for Parkinson’s Disease- A Look Forward and Backward. Neurology 2016; 86: S3-S12
- Ahlskog JE, Muenter MD: Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001; 16: 448-458.
- Chaudhuri RK, Poewe W, Brooks D: Motor and nonmotor complications of levodopa: phenomenology, risk factors, and imaging features. Mov Disord. 2018;33: 909-919.
- Hung SW, Adeli GM, Arenovich T, et al: Patient perception of dyskinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2010;81: 1112.
- Chou KL, Stacy M, Simuni T, et al: The spectrum of “off” in Parkinson’s disease: what have we learned over 40 years? Parkinsonism and Related Disorders 2018; 51: 9-16.
- Politis M, Wu K, Molloy S, et al: Parkinson’s disease symptoms: the patient’s perspective. Mov Disord. 2010;25: 1646-1651.
- Stocchi F, Antonini A, Barone P, DEEP study group, et al: Early DEtection of wEaring off in Parkinson disease: the DEEP study. Parkinsonism Relat Disord. 2014 Feb;20(2): 204-211.
- Poewe W, Antonini A, Zijlmans JC, et al: Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clin Interv Aging. 2010 Sep 7;5: 229-238
- Olanow CW, Kieburtz K, Rascol O, et al: for the STRIDE-PD investigators. Factors predictive of the development of levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord 2013; 28: 1064-1071.
- Fox SH, Katzenschlager R, Lim SY, et al: International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33: 1248-1266.
- Pahwa R, Tanner CM, Hauser RA, et al: ADS-5102 (Amantadine) Extended-Release Capsules for Levodopa-Induced Dyskinesia in Parkinson Disease (EASE LID Study): A Randomized Clinical Trial. JAMA Neurol. 2017 Aug 1;74(8): 941-949.
- Aradi SD, Hauser RA: Medical Management and Prevention of Motor Complications in Parkinson’s Disease. Neurotherapeutics. 2020 Aug 5. doi: 10.1007/s13311-020-00889-4
- Müller T: Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Drugs. 2015 Feb;75(2): 157-74.
- Lees AJ: Evidence-based efficacy comparison of tolcapone and entacapone as adjunctive therapy in Parkinson’s disease. CNS Neurosci Ther. 2008 ;14(1): 83-93.
- Scott LJ: Opicapone: a review in Parkinson’s disease. Drugs. 2016 Sep;76(13): 1293-1300.
- Rocha JF, Falcão A, Santos A, et al: Effect of opicapone and entacapone upon levodopa pharmacokinetics during three daily levodopa administrations. Eur J Clin Pharmacol. 2014 Sep;70(9): 1059-1071.
- Fabbri M, Ferreira JJ, Lees A, et al: Opicapone for the treatment of Parkinson’s disease: A review of a new licensed medicine. Mov Disord. 2018 Oct;33(10): 1528-1539.
- Lees AJ, Ferreira J, Rascol O, et al: Opicapone as Adjunct to Levodopa Therapy in Patients With Parkinson Disease and Motor Fluctuations: A Randomized Clinical Trial. JAMA neurology. 2017;74(2): 197-206.
- Ferreira JJ, Lees A, Rocha JF, et al: Bi-Park 1 investigators. Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol. 2016 Feb;15(2): 154-165.
- Ferreira JJ, Lees A, Rocha JF, et al: Long-term efficacy of opicapone in fluctuating Parkinson’s disease patients: a pooled analysis of data from two phase 3 clinical trials and their open-label extensions. Eur J Neurol. 2019 Jul;26(7): 953-960.
- Ferreira JJ, Lees A, Poewe W, et al: Effectiveness of Opicapone and switching from Entacapone in Fluctuating Parkinson’s Disease. Neurology 2018; 90: e1849-e1857.
- Lees A, Ferreira JJ, Rocha JF, et al: Safety Profile of Opicapone in the Management of Parkinson’s Disease. J Parkinsons Dis. 2019;9(4): 733-740.
- Scott LJ. Opicapone: A Review in Parkinson’s Disease. CNS Drugs. 2021 Jan;35(1): 121-131.
- Reichmann H, Lees A, Rocha JF, et al: OPTIPARK investigators. Effectiveness and safety of opicapone in Parkinson’s disease patients with motor fluctuations: the OPTIPARK open-label study. Transl Neurodegenerate. 2020 Mar 4;9: 9.
- Poewe W, Deuschl G, Seppi K, et al: Parkinson’s disease – therapy. In: G.Deuschl, WH Oertel, W Poewe: Parkinson syndromes and other movement disorders, 2nd edition, Thieme Stuttgart, 2020, 109-157.
InFo NEUROLOGY & PSYCHIATRY 2021; 19(2). Published 3/17/21 (ahead of print).