As part of a 2019 revision of the Swiss vaccination schedule, pneumococcal vaccination for under-5s was added to the list of basic vaccinations. This reassessment is based on an evaluation by the Federal Commission for Immunization Issues (EKIF) and the Federal Office of Public Health (FOPH).
The rationale for this adjustment includes the epidemiologic development of pneumococcal disease in Switzerland and the proven efficacy of this vaccination in protecting individuals and the Swiss population from invasive pneumococcal disease (IPE) [1]. A recommendation for pneumococcal vaccination as a supplemental vaccination for infants has existed since 2006 for protection of children against the most common types of invasive pneumococcal disease [1]. IPEs are common and severe infectious diseases, which usually lead to hospitalization [1,2]. Streptococcus pneumoniae (pneumococcus) infections are among the most common causes of pneumonia in adults. Pneumomias are characterized by high morbidity and lethality.
Epidemiological evidence for efficacy
Risk groups for serious health consequences of IPE are primarily children under 5 years of age, the elderly, persons with immunodeficiency or certain preexisting conditions (e.g., severe heart failure, COPD). PCV13 vaccination has been shown to be highly effective in protecting children under 5 years of age from IPE, thereby reducing the burden of disease [2,3]. Furthermore, an 80% vaccination coverage rate in children younger than 2 years of age has been shown to provide indirect protection against invasive pneumococcal disease in other age groups as well (“herd immunity”). Additional public health benefits of PCV13 vaccination include: positive impact on noninvasive pneumococcal disease, observed decrease in resistant pneumococcal strains in Switzerland, reduction in antibiotic use due to prevented pneumococcal infections. Potential cost impacts were also considered in the evaluation and were estimated to be insignificant [1]. Regarding vaccination schedule, there are no changes; the basic vaccination of infants (without risk factors) includes 3 vaccinations at 2, 4, and 12 months of age [2,4,5].
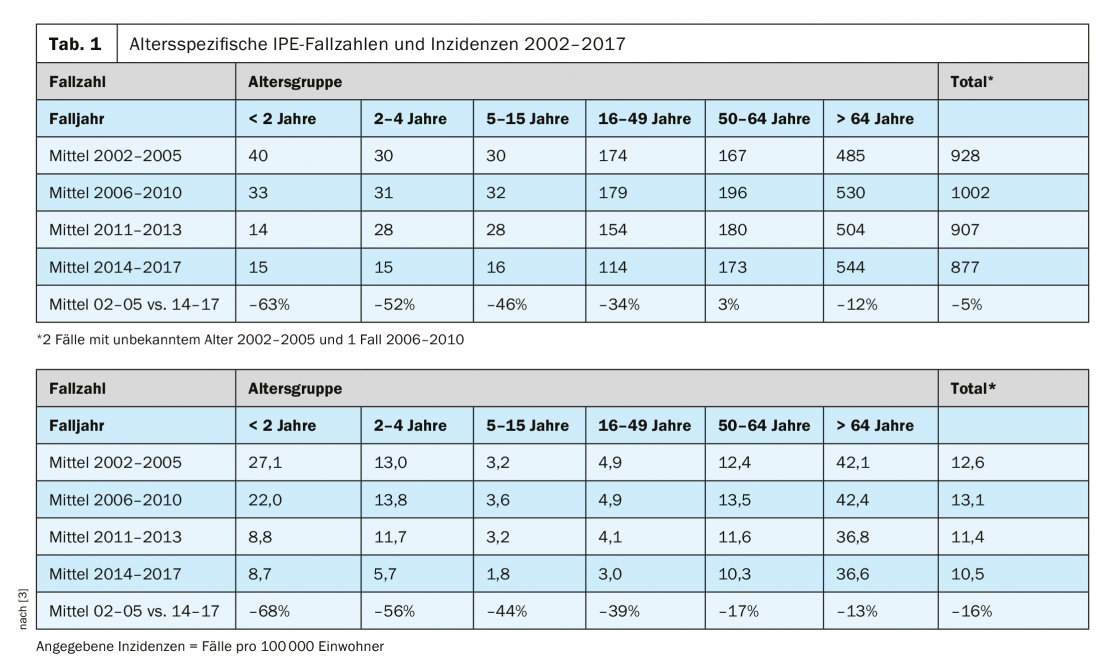
PCV13: vaccination coverage rates increased and incidences reduced
PCV13 is a 13-valent vaccine that replaced the then-used 7-valent pneumococcal conjugate vaccine in 2011 and correlates positively with vaccination coverage rates [1]. Thus, vaccination coverage rates of 75% in 2011-2013 and 80% in 2014-2016, respectively, represent a significant increase from 2008-2010, when this rate was only 37% [1]. A comparison of the incidences of IPE in the period 2002-2005 with the period 2014-2017 shows a significant decrease in all age groups, although this is more pronounced among the <2-year-olds (minus 68%) was the highest ( Tab. 1). A reduction of 56% was recorded in 2-4-year-olds (Tab. 1), with this downward trend coinciding with the switch to PCV13, the extension of the vaccination age for catch-up vaccination, and the increase in vaccination coverage to 80% [3]. In children younger than 5 years, the significantly reduced IPE disease burden is due to prevention of IPE-causing serotypes covered by PCV [3]. This is congruent with the high efficacy of PCV vaccination against IPE caused by vaccine serotypes (77-94%) demonstrated in clinical trials [7–9].
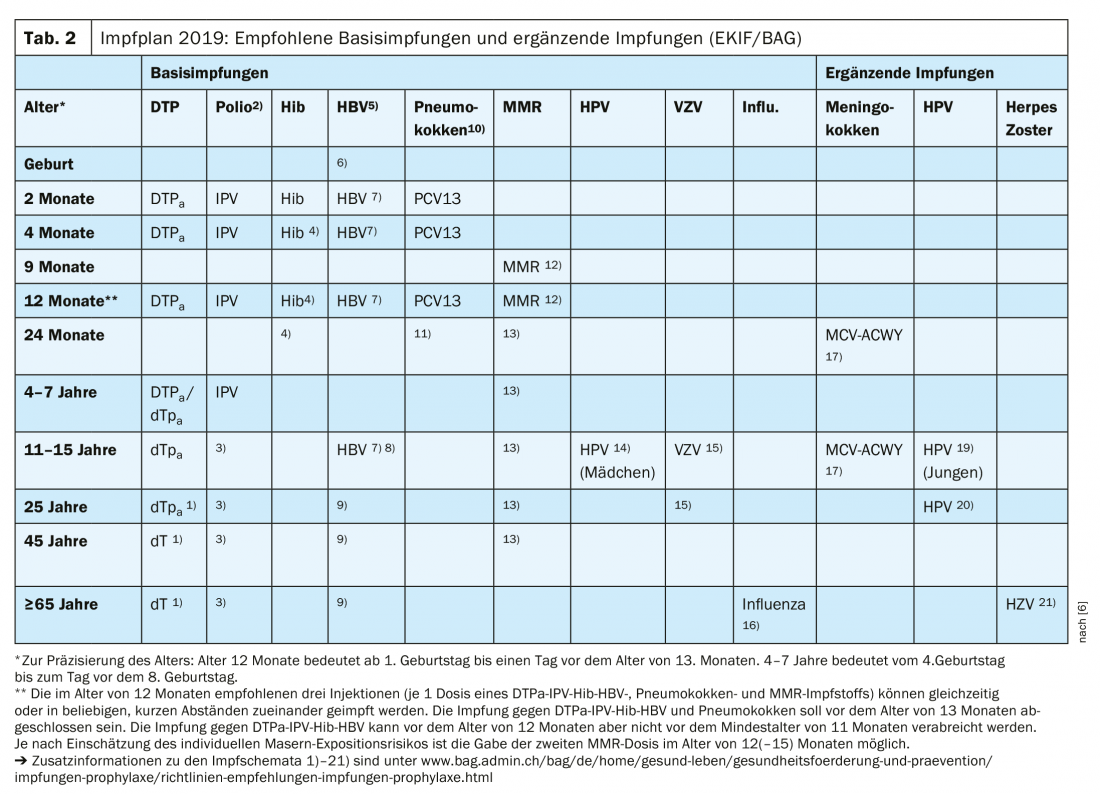
Updates vaccination schedule 2019 at a glance
The main adjustments are as follows [2,10]: The vaccination schedule for infants has been simplified, 3 vaccine doses are now recommended at 2, 4 and 12 months of age (“2+1” vaccination schedule instead of the previous “3+1” vaccination schedule). This infant vaccination regimen, reduced by one dose, is already used in many European countries with positive outcomes in terms of efficacy. It is recommended that at 12 months of age, all three recommended vaccinations be administered during one consultation (1 dose each of a DTPa-IPV-Hib- HBV, pneumococcal, and MMR vaccine). Alternatively, the injections can be divided into two consultations at intervals as short as desired. Infants who, according to previous recommendations, have been treated with a 3rd DTPa-IPV-Hib(-HBV) vaccine dose at 6 months of age must continue to receive a 4. dose received from the age of 12 months (minimum distance from the 3rd dose: 6 months). The recommended basic and supplementary vaccinations according to the current version of the vaccination schedule are shown in Table 2 .
Literature:
- FOPH: Bulletin 13, March 25, 2019. www.bag.admin.ch
- BAG: Guidelines and recommendations on vaccinations and prophylaxis, www.bag.admin.ch/bag/de/home/gesund-leben/gesundheitsfoerderung-und-praevention/impfungen-prophylaxe/richtlinien-empfehlungen-impfungen-prophylaxe.html
- FOPH, Federal Commission for Immunization Questions (EKIF): Pneumococcal vaccination of children under 5 years of age newly recommended as basic vaccination. Bull BAG 2019; no. 13: 32-34. www.bag.admin.ch
- Federal Office of Public Health and Federal Commission on Immunization. Recommendations for pneumococcal vaccination in children younger than 5 years: Switch from 7- to 13-valent conjugate vaccine. Bull BAG 2010; no. 51: 1202-1205.
- FOPH: Supplement to Supplement XVII – Fewer vaccine doses, same benefit: Reduction of pneumococcal vaccination schedule in healthy children younger than 2 years. Bull BAG 2006; no. 21: 409-411.
- BAG: Synopsis Swiss Vaccination Plan 2019, www.bag.admin.ch/bag/de/home/gesund-leben/gesundheitsfoerderung-und-praevention/impfungen-prophylaxe/richtlinien-empfehlungen-impfungen-prophylaxe.html
- FOPH, Federal Commission for Immunization Questions (EKIF): Pneumococcal vaccination in children under 5 years of age. Guidelines and Recommendations (formerly Supplementum XVII). Bern: BAG, 2005.
- Klugman KP, Black S, Dagan R, et al: (2013) Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In: Plotkin SA, Orenstein WA, Offit PA (eds): Vaccines 6th Edition, Philadelphia: Elsevier Saunders, 504-541.
- Andrews NJ, et al: Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. The Lancet. Infectious diseases 2014; 14(9): 839-846.
- FOPH, Federal Commission for Immunization Questions (EKIF): The new “2+1 vaccination scheme” for the basic vaccination of infants against diphtheria, tetanus, pertussis, poliomyelitis, H. influenzae type b and hepatitis B: one dose less. Bull BAG 2019; no. 13: 18-22.
HAUSARZT PRAXIS 2019; 14(9): 27-28