Improvement in clinical endpoints was seen with secukinumab treatment regardless of latency since initial psoriatic arthritis diagnosis, with patients diagnosed less than one year ago showing a slightly better response than those diagnosed more than two years ago. In another post-hoc analysis of a data pool of the same four studies, the efficacy of secukinumab as first-line therapy was shown to be superior compared to patients pretreated with anti-TNFα.
Psoriatic arthritis (PsoA) can progress rapidly if left untreated, leading to irreversible damage within two years of initial diagnosis [1]. The selective interleukin (IL)-17 inhibitor secukinumab showed rapid and sustained symptom improvement in the phase III FUTURE 1-5 trials. The median duration since PsoA diagnosis in these studies was 6-7 years [2–6]. To gain a better understanding regarding the effects of prior therapeutic intervention in patients with PsoA, an evaluation of secukinumab treatment as a function of duration since initial PsoA diagnosis was performed [7]. To this end, pooled data (n=1803) from the FUTURE 2-5 randomized-controlled trials were analyzed*. Included patients received either secukinumab (s.c.) 300 mg or 150 mg as loading dose, secukinumab 150 mg without loading dose, or placebo. The patients were divided into two groups: group 1 included patients who had been diagnosed with PsoA ≤1 year ago, and group 2 included patients who had been diagnosed with PsoA >2 years ago. Treatment response was assessed by, among other things, changes in ACR20/50/70 as well as PASI75/90/100 after 16 weeks**. Quality of life and disease activity outcome parameters were also recorded.
* FUTURE 2 (NCT01752634), FUTURE 3 (NCT01989468), FUTURE 4 (NCT02294227) and 5 (NCT02404350).
** Non-Responder Imputation; no correction regarding multiple comparisons.
Higher symptom improvement with lower treatment latency.
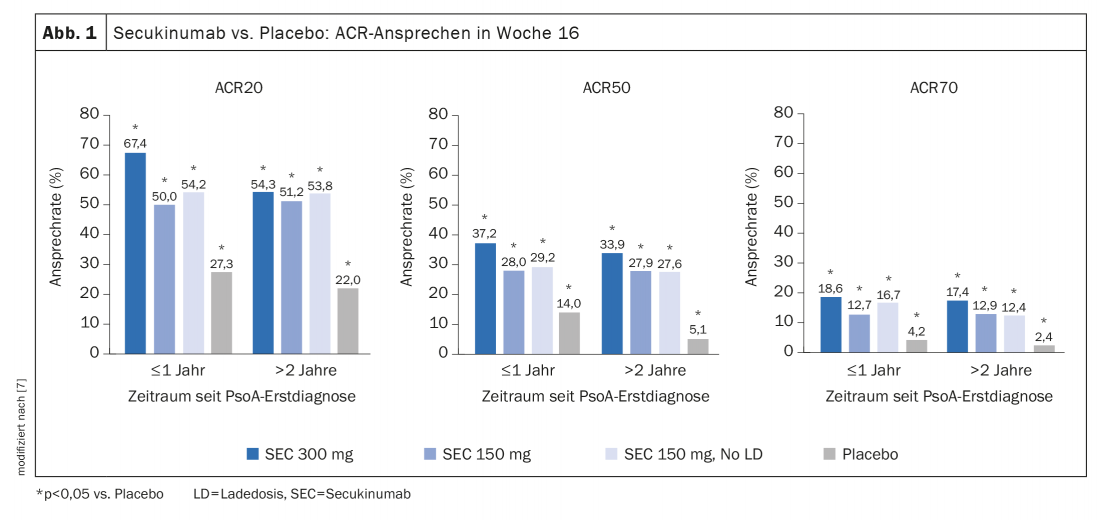
Overall, 419 patients (23.2%) had a first PsoA diagnosis ≤1 year ago at the start of treatment with secukinumab, and 1384 (76.8%) had a treatment latency >of 2 years [7]. At baseline, most relevant patient characteristics of these two groups were comparable. At week 16, ACR20/50/70 response rates proved higher with secukinumab treatment compared with placebo, regardless of latency in time since PsoA diagnosis (Fig. 1), with secukinumab 300 mg associated with higher ACR response rates than secukinumab 150 mg. In general, ACR response rates were slightly higher in patients with a treatment latency ≤1 year, particularly in those receiving secukinumab 300 mg. Secukinumab also proved superior to placebo in terms of other outcome parameters such as enthesitis, dactylitis, skin psoriasis and nail psorisais. A higher proportion of patients with a secukinumab treatment latency ≤1 year compared with the group with an >initial PsoA diagnosis 2 years earlier had no swollen joints and no tender joints at all (“swollen joint count” = 0, “mean tender joint count” = 0) and a CRP ≤10 mg/L. Patients with lower treatment latency also performed better on subscores for psychological well-being in the SF-36 (“Short Form-36 of the Health Survey”). The most common adverse events in patients treated with secukinumab (time since diagnosis ≤1 year and >2 years, respectively) were nasopharyngitis (8.3% and 6.1%, respectively), headache (6.2% and 3.6%, respectively), and upper respiratory tract infections (5.1% and 4.7%, respectively).
Data on secukinumab as first-line treatment
First, a further post-hoc analysis of pooled data (n=2049) from the FUTURE 2-5 trials showed that the efficacy of secukinumab was superior to placebo in all of the domains of clinical PsoA manifestation defined by the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) (peripheral arthritis, axial involvement, enthesitis, dactylitis, skin psoriasis, nail involvement) [8]. Second, the clinical response was higher in anti-TNFα-naive patients compared to those previously treated with TNFα inhibitors. These results provide an argument for using secukinumab as a first-line treatment option.
Literature:
- Kane D, et al: Rheumatology (Oxford) 2003; 42: 1460-1468.
- Mease PJ, et al. RMD Open 2018; 4: e000723.
- McInnes IB, et al. Lancet 2015; 386: 1137-1146.
- Nash P, et al. Arthritis Res Ther 2018; 20: 47.
- Kivitz A, et al. Rheumatol Ther 2019; 6(3): 393-407.
- Mease PJ, et al. Ann Rheum Dis 2018; 77: 890-897.
- Ritchlin C, et al: Arthritis Rheumatol 2020; 72 (suppl 10). https://acrabstracts.org/abstract/efficacy-of-secukinumab-treatment-in-patients-with-early-psoriatic-arthritis-a-pooled-analysis-of-4-phase-3-studies (last accessed Sep 20, 2021).
- Orbai AM, et al: Rheumatol Ther 2021: 1223-1240.
DERMATOLOGIE PRAXIS 2021; 31(5): 57-58