The SURPASS study program investigated the efficacy and safety of the dual GIP/GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes. An exploratory analysis of the Phase III SURPASS 1-4 studies shows that normoglycemia was achieved in a significant proportion of participants without increasing the risk of hypoglycemia. This was accompanied by an improvement in general metabolic health. These results were published in the journal Diabetes Care .
Tirzepatide is the first approved dual GIP/GLP-1 receptor agonist. The spectrum of action of tirzepatide includes an increase in insulin secretion, a reduction in glucagon and glucose levels, a delay in gastric emptying and a reduction in body weight. The effects are based on agonism at the GIP and GLP-1 receptors. In Switzerland, tirzepatide (Mounjaro®) has been approved for the treatment of type 2 diabetes since 2022; in the EU and some other regions, the indication for obesity has been extended [1,2]. The medicine is administered once a week as a subcutaneous injection.
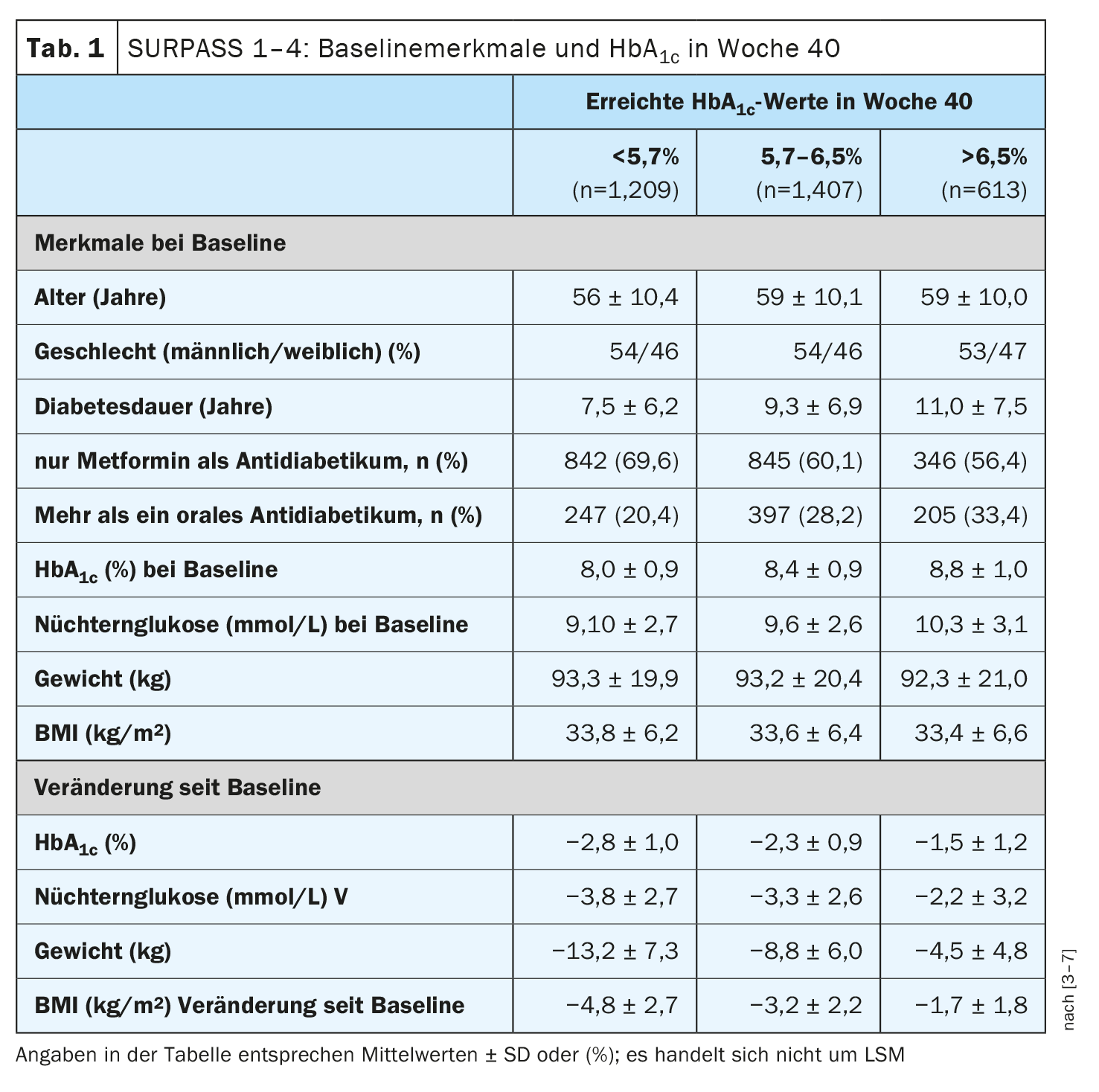
Rosenstock et al. analyzed the values by HbA1c category (<5.7%, 5.7-6.5% and >6.5%) of participants who had taken at least 75% of the treatment doses of tirzepatide without rescue medication in SURPASS studies 1-4 (n=3229) [3–7]. To assess the impact of patient characteristics on achieving an HbA1c <5.7%, odds ratios were calculated using logistic regression models with tirzepatide as a covariate. It was found that the participants treated with tirzepatide who achieved an HbA1c <5.7% were slightly younger at the start of the study, had a shorter duration of diabetes and a lower HbA1c value (Table 1). Over the course of the study, these participants also showed greater improvements in HbA1c, body weight, waist circumference, blood pressure, liver enzymes and lipid parameters, without any increase in the risk of hypoglycemia.
Baseline to week 40: important results at a glance
The group of patients who achieved an HbA1c value <5.7% at week 40 showed a mean HbA1c change of -3.0% compared to -2.3% in those whose HbA1c value was 5.7-6.5% and compared to -1.1% in patients with an HbA1c value >6.5% (least mean squares [LSM]; p<0.001 in each case). The group with an HbA1c value <5.7% achieved a mean body weight reduction of 14.1%, the group with an HbA1c of 5.7-6.5% of 9.6% and in the group with HbA1c >6.5% the weight loss was 5.1% (LSM, p<0.001 in each case). A higher baseline HbA1c (each 1% increase) and fasting glucose (each 50 mg/dl increase) was associated with a lower likelihood of achieving an HbA1c <5.7% at week 40 (42% and 24%, respectively). In addition, the probability of achieving an HbA1c value <5.7% decreased by 13% and 24% with each 5-year increase in age and diabetes duration, respectively. Only patients receiving metformin had a greater chance of achieving an HbA1c value <5.7%. In terms of adverse event rates, 63% of patients who achieved an HbA1c value <5.7% experienced ≥1 adverse event (AE), while these rates were 62% and 57% in the group of patients who achieved an HbA1c value between 5.7-6.5% and >6.5%, respectively. The most common treatment-related AEs were gastrointestinal complaints (42% vs. 39% and 32% respectively).
Literature:
- Swissmedic: Medicinal product information, www.swissmedicinfo.ch,(last accessed 11.06.2024)
- European Commission, https://ec.europa.eu/health/documents/community-register/2023/
20231211161235/anx_161235_en.pdf, (last accessed 11.06.2024). - Rosenstock J, et al: Achieving Normoglycemia With Tirzepatide: Analysis of SURPASS 1-4 Trials. Diabetes Care 2023; 46(11): 1986-1992.
- Rosenstock J, et al.: Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 2021; 398: 143–155.
- Frías JP, et al; SURPASS-2 Investigators . Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021; 385: 503-515.
- Ludvik B, et al: Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomized, open-label, parallel-group, phase 3 trial. Lancet 2021; 398: 583-598.
- Del Prato S, et al; SURPASS-4 Investigators . Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomized, open-label, parallel-group, multicentre, phase 3 trial. Lancet 2021; 398: 1811-1824.
FAMILY PHYSICIAN PRACTICE 2024; 19(6): 31