In the treatment of fibromyalgia, pregabalin may also be used during concurrent therapy with antidepressants. This is shown by new research findings presented at this year’s ACR Congress in San Diego. In a new dosage form, as once-daily pregabalin “controlled-release” (PGB CR), it also shows good response rates when used alone. In addition, the success of milnacipram appears to be dependent on certain patient factors.
(ag) Lesley M. Arnold, MD, of the University of Cincinnati, Ohio, presented new data on pregabalin. In their study [1], 441 adult, mostly female patients with fibromyalgia participated. Of these, 121 completed the single-blind phase (pregabalin “controlled-release” [PGB CR, once daily = QD] 165 mg/d with an increase within the first three weeks to 495 mg/d), thus had a pain response of ≥50% compared with baseline. With this, they entered the double-blind phase where they received either PGB CR at the optimized dose (330-495 mg/d) or placebo. The primary endpoint was time to no therapeutic response, defined as <30% pain response compared with baseline or discontinuation due to lack of efficacy or side effects.
Overall, therapeutic response failed in 75 cases: 34 of 63 PGB-CR patients in arm 1 and 41 of 58 placebo patients in arm 2, respectively. The median time to this event was 58 days for the PGB-CR group and 22 days for the placebo group (p=0.021). Regarding the secondary endpoints (pain relief, general assessment, functional status, fatigue, and sleep), there were no significant differences, except for “benefit of treatment,” where significantly more patients reported a “great benefit” with PGB CR. The most common adverse events in the double-blind phase were dizziness, peripheral edema, and insomnia (under PGB CR) or dizziness, drowsiness, dry mouth, and peripheral edema (under placebo). Side effects were predominantly mild to moderate. One severe adverse event (glossitis) was considered to be associated with PGB-CR treatment.
“Overall, it can be concluded from the results that PGB CR is effective in prolonging the time to treatment response and reducing the number of such events. It was also well tolerated and the safety profile was consistent with that of PGB,” Arnold concluded her presentation [1].
How do comorbidities affect therapy?
Fibromyalgia is a chronic disease that usually involves pain all over the body. Furthermore, affected persons suffer from touch or pressure dolences affecting joints and muscles, as well as fatigue, sleep disturbances or memory problems. It is not uncommon for mood swings to occur, for example, there is an association with depression. “About 50-70% of patients have a lifetime history of depression and about 25% were already taking antidepressants,” Arnold explained.
Their study [2] shows that individuals with fibromyalgia taking antidepressants can successfully control their pain with pregabalin. Pregabalin is approved for the treatment of fibromyalgia in many states. To date, however, studies had not examined the additional use of antidepressants in therapy. “Yet depression is common in this population: Many people who come to the doctor with such pain are already taking antidepressants. So our study is the first to investigate the safety and efficacy of pregabalin in these patients,” Arnold says.
Pregabalin and antidepressants
A 14-week double-blind, placebo-controlled crossover study [2] enrolled 197 patients with a mean age of 50 years, predominantly female gender, and a documented diagnosis of depression who were taking stable doses of selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs). They were randomized to receive either pregabalin or placebo. There were two six-week treatment periods with an intervening two-week break (wash-out). Patients on pregabalin switched to placebo after the first period, and the second arm received reverse pregabalin at that time.
The initial dose of pregabalin was 150 mg/d and was increased to 300 to 400 mg/d within the first three weeks, until the end of the treatment period. This showed that pregabalin was associated with significantly less pain: the initial pain score was 6.7. It was reduced to 4.84 with pregabalin and to 5.45 with placebo.
Side effects occurred in 77.3% of pregabalin patients and in 59.9% of placebo patients. For pregabalin, dizziness and lightheadedness were mostly reported. Four serious adverse events occurred but were not associated with treatment, according to the authors [2].
“The study shows that pregabalin can be used safely and effectively in depressed patients with fibromyalgia and on antidepressant therapy,” Arnold concluded.
What are the benefits of milnacipran?
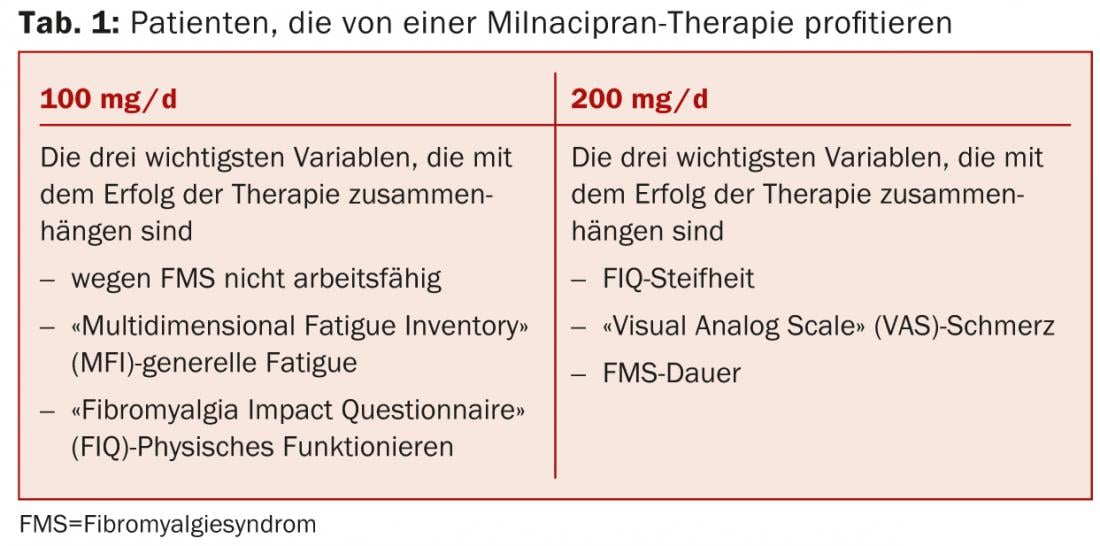
Olivier Vitton of Cypress Bioscience, San Diego, presented the results of an analysis [3] that examined which baseline characteristics of fibromyalgia patients were associated with the success of milnacipran (MLN) therapy in multiple clinical trials. Data from three placebo-controlled phase III trials (totaling more than 2500 patients) were examined. The results in Table 1 suggest that targeted patient selection is useful for personalized therapy with the SNRI milnacipran. However, the drug is not approved for the treatment of fibromyalgia in Switzerland, as it is throughout Europe.
Source: ACR/ARHP Annual Meeting, October 25-26, 2013, San Diego.
Literature:
- Arnold LM, et al: Once Daily Controlled-Release Pregabalin In Fibromyalgia Patients: A Phase 3 Double-Blind, Randomized Withdrawal, Placebo-Controlled Study. ACR Abstract #2850.
- Arnold LM, et al: Efficacy and Safety of Pregabalin in Patients with Fibromyalgia and Co-Morbid Depression Receiving Concurrent Antidepressant Therapy: A Randomized, 2-Way Crossover, Double-Blind, Placebo-Controlled Study. ACR Abstract #L6.
- Vitton O, et al: Which Baseline Characteristics Influence The Response To Milnacipran In Patient With Fibromyalgia? ACR Abstract #1109.











