Estimates showed that approximately 1.8 million neurons are lost per minute in the course of ischemic damage caused by reduced blood supply. So in acute cerebral stroke, things have to happen quickly. Emergency imaging diagnostics enable an early diagnosis and have a decisive influence on therapy.
Every 30 minutes, someone in Switzerland suffers a stroke. Approximately 25% of those affected die as a direct result, and about one-third remain disabled to varying degrees throughout their lives [1]. In 87% of cases, stroke is the result of an acutely reduced blood supply in the context of a thromboembolic vessel occlusion or a hemodynamically relevant vascular stenosis, and in 13% the result of an intracranial or subarachnoid hemorrhage [2]. Estimates indicated that approximately 1.8 million neurons are lost per minute in the course of ischemic injury caused by reduced blood supply [3]. In the case of acute cerebral stroke, therefore, things have to move quickly, and immediate diagnostic clarification and therapy are required. Emergency imaging diagnostics enable an early diagnosis and have a decisive influence on therapy.
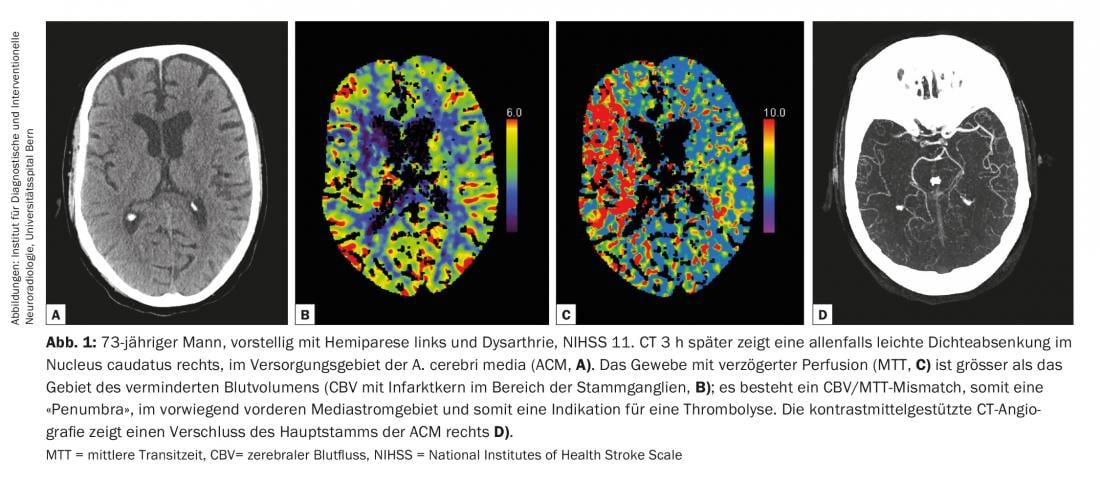
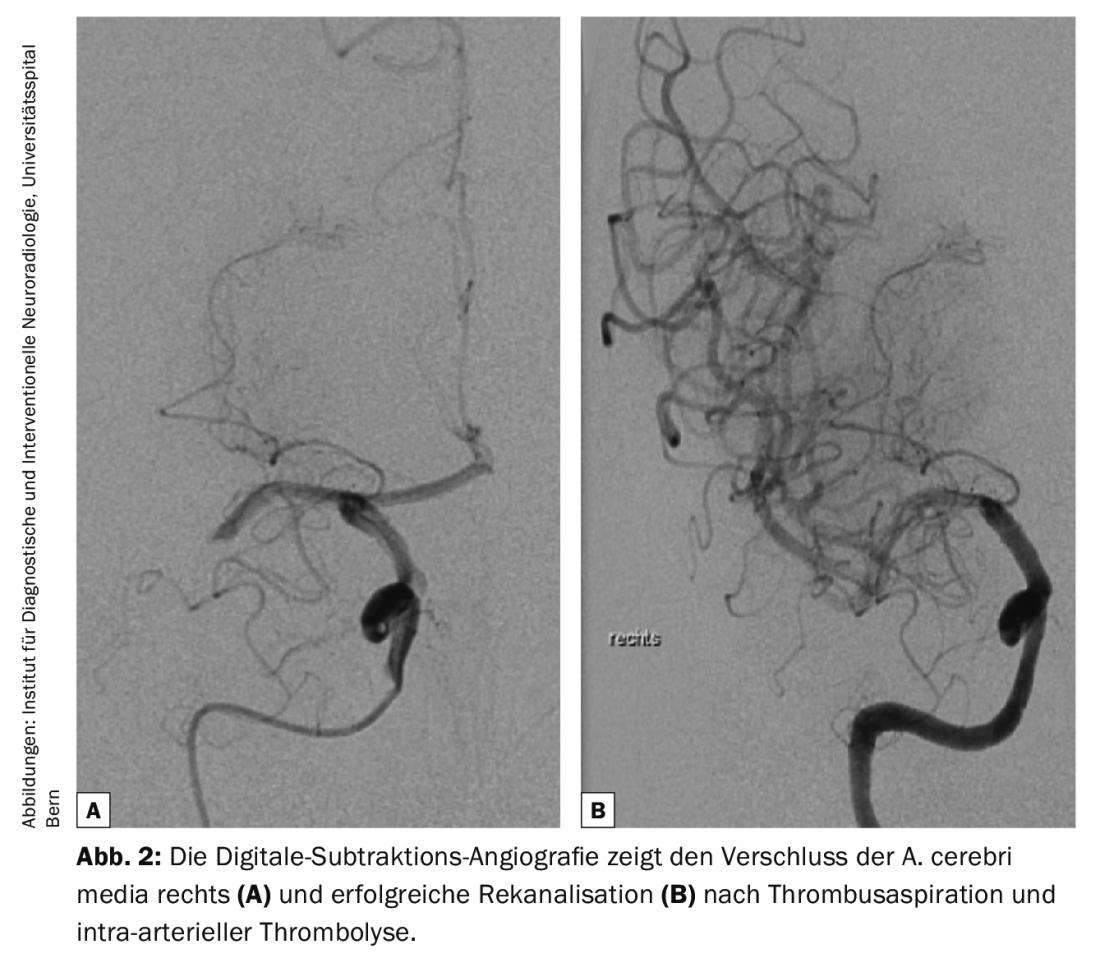
Diagnostic guidelines on cerebrovascular disease are continuously updated by the American College of Radiology (ACR) and the American Heart Association/American Stroke Association (AHA/ASA) to reflect current knowledge [2,4]. All modalities from computed tomography (CT) and magnetic resonance imaging (MRI) to ultrasound (US) and digital subtraction angiography (DSA) have their place in the evaluation of cerebrovascular diseases [2] (Fig. 1-3). In the setting of acute stroke diagnosis, CT and MRI are predominantly used. The following questions should be answered reliably and quickly:
- Intracranial hemorrhage?
- Vessel closure?
- Infarct core?
- Potentially salvageable brain parenchyma (“ischemic penumbra” or “tissue-at-risk”)?
- “Stroke mimic?
The indication for thrombolysis or interventional therapy is determined by knowing the time window since symptom onset and the present symptoms including the National Institutes of Health Stroke Scale (NIHSS) as well as relevant secondary diagnoses such as cardiovascular risk factors.
Computed tomography (CT)
CT is available around the clock in most hospitals and provides a quick and easy way to rule out acute intracranial hemorrhage. Acute intracranial hemorrhage already presents hyperdense (60-90 HU, thus brighter or denser than healthy brain tissue) on native imaging and is an absolute contraindication for intravenous thrombolysis [4].
In the case of ischemia caused by reduced blood supply (defined as cerebral blood flow [CBF] <10 ml/100 g/min), ion transport fails due to lack of energy and water influx into cells occurs (cytotoxic edema) [5]. The increased water content in the ischemic tissue leads to a decrease in density of the affected area and becomes visible as abolished medullary cortical differentiation (mostly in the insular cortex as “insular ribbon sign”) or as hypodensity in the basal ganglia and medullary camp. This decrease in density should be interpreted as an infarct core in the sense of irreversible parenchymal damage. However, detection of such early and often subtle changes requires a great deal of experience and has an average sensitivity of 66% (range, 20% to 87%) in the first three hours after symptom onset [2].
Also already apparent on native imaging may be an intravascular thrombus that is hyperdense. This so-called “hyperdense artery sign” is highly specific but not very sensitive for vascular occlusion and, like other vascular stenoses or occlusions, can be visualized more precisely with the aid of contrast-enhanced CT angiography. CT angiography allows assessment of all brain afferent arteries from the outflow from the aortic arch to the vertex, thus also allowing assessment of collateral circulation or vascular anomalies that may complicate endovascular intervention.
The potentially salvageable brain parenchyma, also called “penumbra” or “tissue-at-risk”, is the area of critical hypoperfusion (CBF 10-20 ml/100 g/min) [5]. The neurons in the “penumbra” are dysfunctional, although the dysfunction is reversible with timely reperfusion. With persistent occlusion, there is a gradual demise of the “penumbra”. To visualize the infarct core, “penumbra” (“tissue-at-risk”), and adjacent benign oligemia (“tissue-not-at-risk,” CBF >20 ml/100 g/min, norm 60-80 ml/100 g/min), measurement of blood flow by perfusion imaging is required. CT perfusion is a contrast-enhanced dynamic imaging technique that provides information on temporal parameters such as mean transit time (“MTT”), time to peak of the signal intensity-time curve (“TTP”), and time to peak of the so-called residual function (Tmax), respectively, and semi-quantitative parameters such as cerebral blood flow (CBF) and cerebral blood volume (CBV). Delayed perfusion is manifested by prolonged temporal perfusion parameters. Reduced CBF in the “penumbra” results in energy-dependent vasogenic autoregulation; MTT and TTP are prolonged, and CBV is normal or even slightly increased. These compensatory mechanisms are not present in the infarct core, where a decrease in CBV occurs [6]. In CT, the potentially salvageable penumbra is traditionally estimated using a so-called “CBV/MTT mismatch”.
Magnetic resonance imaging (MRI)
In stroke centers, MRI is available 24/7 and, because it provides more additional information, is often preferred to CT. The routine MR examination protocol includes diffusion imaging (DWI), “FLAIR” or T2-weighted spin echo sequence, arterial time-of-flight MR angiography (TOF-MRA), susceptibility-weighted sequence (SWI) or T2* gradient echo sequence (T2*GRE), perfusion MRI, contrast-enhanced angiography of brain-feeding vessels, and T1-weighted sequence after contrast administration.
The sequences sensitive to blood degradation products (SWI or T2*GRE) also allow visualization of any intravascular thrombus. Thrombus length, measured on MRI or CT, has prognostic relevance, with a length greater than 8 mm associated with poor recanalization after i.v. lysis [7].
Increased levels of deoxyhemoglobin result in more prominent MRI signal depression of the cortical, leptomeningeal (“cortical vessel sign”), and deep medullary arteries (“brush sign”) in the susceptibility-weighted sequence, allowing estimation of the hypoperfused area [8].
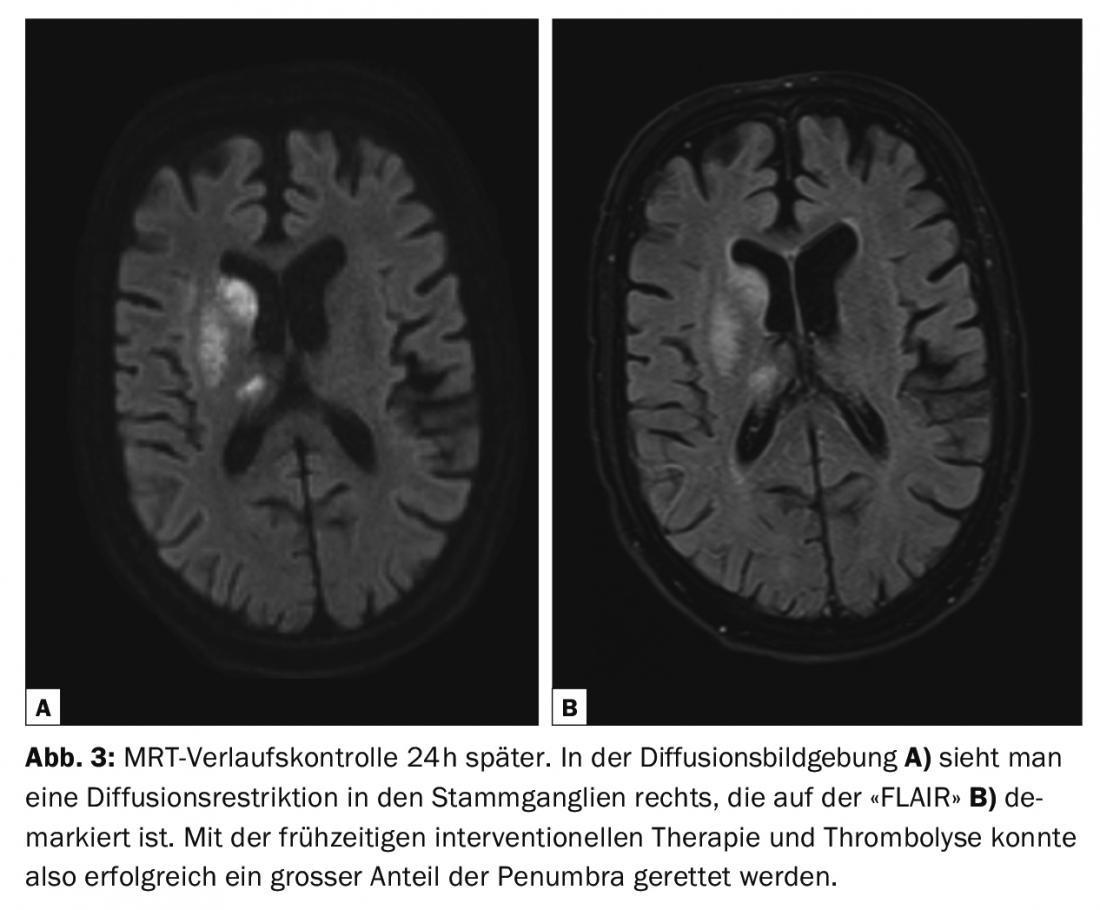
In acute stroke as well as wake-up stroke (time of symptom onset unknown), comparison of diffusion-weighted and “FLAIR” imaging allows temporal classification. The cytotoxic edema, which largely corresponds to the infarct core but also contains some reversible portions from the “penumbra”, is already demarcable after a few minutes on diffusion imaging (sensitivity 88-100%, specificity 95-100%) but only after about 6h post-ictus as signal enhancement on the “FLAIR”/T2 [2,9]. The T1-weighted sequence after contrast administration allows further temporal classification; meningeal hyperemia can be expected in the period between 24 h and 3-4 days, and barrier disruption of the infarcted brain parenchyma in the period between 24 h and several weeks or even months [9].
With TOF angiography, MRI offers the possibility of flow-sensitive vascular imaging without contrast administration and high spatial resolution in addition to contrast-enhanced angiography. MR perfusion, like CT perfusion, also provides information on temporal (MTT, TTP, Tmax) and semi-quantitative (CBV, CBF) parameters after administration of a contrast bolus. In MRI, the ischemic “penumbra” is defined by a difference between the perfusion defect in the MTT or TTP maps and the diffusion defect, as a so-called “diffusion-perfusion mismatch” [2].
Which imaging is targeted?
In the current 2018 guidelines [4], the American Heart and Stroke Associations (AHA/ASA) primarily recommend native cranial CT to rule out intracranial hemorrhage in any patient with suspected acute stroke. Imaging of the extracranial and intracranial carotid and vertebral arteries is appropriate in potential candidates for mechanical thrombectomy to plan the procedure but should not delay the administration of intravenous alteplase if indicated.
Furthermore, in patients with large proximal occlusions in the anterior circulation and in the time window between 6-24 h since symptom onset, perfusion imaging by CT or MRI is recommended for indication of possible mechanical thrombectomy. Nowadays, imaging can be performed at high speed. In addition, candidates for thrombectomy cannot be identified with sufficient reliability clinically. Therefore, we use an MRI protocol in the acute stroke patient with perfusion and diffusion imaging as well as imaging of the brain-supplying vessels. To administer the bolus of i.v. lysis, the MRI scan may be briefly interrupted after bleeding has been ruled out. In clinical practice, the choice of imaging is mainly dependent on the available infrastructure and experience in the respective center.
However, initial assessment by MRI offers several advantages: With DWI, MRI offers very early sensitive visualization of the ischemic brain parenchyma and is clearly superior to CT in this regard. MRI is also clearly superior to CT for clinical suspicion of infratentorial infarcts in the brainstem and cerebellum [10]. Patients with neurological deficits that are difficult to classify clinically can thus be reliably differentiated into patients with acute ischemia or with a “stroke mimic” such as hemiplegic migraine, Todd’s palsy, or cranial nerve disease [11]. For detection of other rare causes of cerebral stroke, such as vascular dissection, fibromuscular dysplasia, or sinus or cerebral venous thrombosis, MRI is preferred. The superior temporal classification of the infarct on MRI allows a more differentiated decision regarding interventional therapy. The potentially harmful effects of ionizing radiation and CT contrast agent containing iodine are another important factor in the appropriate choice of imaging. Disadvantages of MRI are mainly the longer examination time with about 15 min compared to multimodal CT with about 5 min as well as the more difficult feasibility in patients requiring monitoring and agitated patients. If the patient’s MR capability is questionable, a CT should be performed, in this acute situation.
Take-Home Messages
- Therapy-relevant information from imaging is: Exclusion of hemorrhage, detection of ischemia or infarct core, “penumbra” if applicable, vessel occlusion, and thrombus length. CT and MRI answer these questions and are used in clinical practice mainly depending on the existing infrastructure and experience of the respective hospital.
- MRI is very helpful in the evaluation of patients with neurologic deficits that are difficult to classify clinically and suspected brainstem ischemia.
- Patients with regressing neurologic deficits should also receive a complete stroke workup and imaging. Experience has shown that these patients can quickly deteriorate clinically.
- According to the new AHA/ASA guidelines, a CT or MRI stroke protocol should include native series for hemorrhage exclusion and/or detection of ischemia, as well as vascular imaging. However, the latter should not delay intravenous thrombolysis if indicated.
Literature:
- Federal Office of Public Health, Quality Indicators of Swiss Acute Hospitals, Quality Indicators Number of Cases: B1.1.M HD Stroke all forms (age >19), 2015 (www.bag.admin.ch/bag/de/home/service/zahlen-fakten/zahlen-fakten-zu-spitaelern/qualitaetsindikatoren-der-schweizer-akutspitaeler/qualitaetsindikatoren-fallzahl.exturl.html/aHR0cDovL3d3dy5iYWctYW53LmFkbWluLmNoLzIwMTZfdGFnbG/FiLzIwMTZfc3BpdGFsc3RhdGlzdGlrL3BvcnRhbC5waHA_cD1x/aWZhbGx6Jmxhbmc9ZGUmYmFza2V0PSU3Q2IxLjElN0MwJnF5PT/IwMTY=.html).
- DeLaPaz RL, et al: ACR Appropriateness Criteria on Cerebrovascular Disease. J Am Coll Radiol 2011; 8(8): 532-538.
- Saver JL: Time is brain – quantified. Stroke 2006; 37: 263-266.
- Powers WJ, et al: 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American heart Association / American Stroke Association. Stroke 2018; 49: e46-e110. doi: 10.1161/STR.0000000000000158.
- Astrup J, et al: Thresholds in cerebral ischemia – the ischemic penumbra. Stroke 1981; 12(6): 723-725.
- Knash M, et al: Low cerebral blood volume is predictive of diffusion restriction only in hyperacute stroke. Stroke 2010; 41(12): 2795-2800.
- Riedel CH, et al: The importance of size: successful recanalization by intravenous thrombolysis in acute anterior stroke depends on thrombus length. Stroke 2011; 42(6): 1775-1777.
- Morita N, et al: Ischemic findings of T2*-weighted 3-tesla MRI in acute stroke patients. Cerebrovasc Dis 2008; 26(4): 367-375.
- Allen LM, et al: Sequence-specific MR imaging findings that are useful in dating ischemic stroke. Radiographics 2012; 32(5): 1285-1297.
- Wintermark M, et al: Imaging Recommendations for Acute Stroke and Transient Ischemic Attack Patients: A Joint Statement by the American Society of Neuroradiology, the American College of Radiology and the Society of NeuroInterventional Surgery. J Am Coll Radiol 2013; 10(11): 828-832.
- Birenbaum D, Bancroft LW, Felsberg GJ: Imaging in stroke. West J Emerg Med 2011; 12(1): 67-76.
CARDIOVASC 2018; 17(2): 16-19