Nowadays, chronic prurigo is defined as a disease in its own right. Prurigo nodularis in particular is very distressing for those affected. Local therapies are not effective in all patients, but there are several biologics and JAK inhibitors that have shown promising results in trials. Dupilumab and nemolizumab in particular are great hopefuls, especially since both biologics have already received FDA approval for this indication.
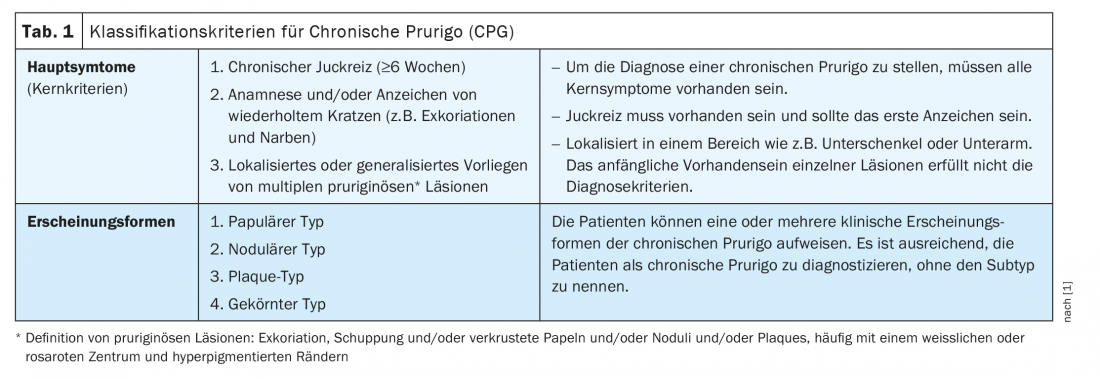
Chronic prurigo (CPG) can be diagnosed when chronic pruritus persists for at least 6 weeks, there is evidence of repeated scratching (e.g., excoriations and scars), and multiple localized or generalized pruriginous* lesions are present. These classification criteria were established in 2018 by a task force of the European Academy for Dermatology and Veneorology (EADV) (Table 1) [1]. CPG is often associated with a significant burden of disease. “Patients suffer and have an impaired quality of life,” said Prof. Dr. med. Dr. h.c. Andreas Wollenberg, Department of Dermatology and Allergology LMU Munich and Department of Dermatology, Vrije Universiteit Brussels [2]. CPG results from neuronal sensitization to itch and the development of an itch-scratch cycle. Etiologically, dermatologic, systemic, neurologic, or psychiatric/psychosomatic factors may be considered. However, prurigo may also be multifactorial or have an unexplained etiology [1]. According to expert consensus, CPG is considered an umbrella term for papular, nodular, and other manifestations (Table 1) [1]. In addition to the aforementioned mandatory core criteria, secondary criteria were defined, including impaired quality of life, sleep disturbance, depression, anxiety, and helplessness.
Local therapy: TCS and TCI are still the standard of care
Regarding topical corticosteroids (TCS), Prof. Wollenberg prefers agents of strength class 4 (e.g. clobetasol proprionate) [2]. When topical corticosteroids are used under occlusion, this strength class is also advisable. Topical calcineurin inhibitors (TCI) can be used as an alternative to TCS. One advantage of TCIs is that they do not lead to the steroid-typical side effects. Tacrolimus ointment has been shown to be more effective compared with pimecrolimus cream in three six-week multicenter, investigator-blinded randomized trials involving a total of 1065 patients and should be preferred [13]. Tacrolimus could also be used under occlusion, he said, but this only made sense for a small number of lesions. Intralesional corticosteroids may also be helpful if only a few lesions are present. Itching can be reduced as a result, but the cosmetic outcome is sometimes suboptimal, the speaker said. The use of topical vitamin D3 analogues is also justified – in some cases, good results can be achieved with them, reports Prof. Wollenberg. Light therapy (PUVA, narrowband UVB) may eventually be used in combination with topical TCS. And for a small limited area, treatment with excimer laser (around 300 nm) is worth trying. Cryotherapy is also a possibility, although the lecturer is not a supporter of this therapy option, as experience has shown that the cosmetic outcome leaves much to be desired [2].
Biologics are on the rise: Dupilumab and Nemolizumab convince
Dupilumab, a monoclonal antibody already approved for other indications in Europe, exerts anti-inflammatory effects by inhibiting the IL4/IL13 signaling cascade. In a case series by researchers at the Karolinska Institute, Stockholm, dupilumab resulted in significant symptom reduction in patients with prurigo nodularis (PN), and in the randomized, placebo-controlled PRIME-2 trial, 37.2% of dupilumab-treated patients achieved a clinically meaningful reduction in itch since baseline at week 12 versus 22.0% in the placebo group (p=0.0216) [2–5]. At week 24, the corresponding scores were 57.7% in the dupilumab arm versus 19.5% in the placebo arm (p<0.0001), thus nearly three times as many study participants in the verum condition achieved clinically meaningful itch reduction at approximately six months (Fig. 1) [2,4,5]. Appearance-free or near-appearance-free skin at week 24 was seen in 44.9% of dupilumab-treated patients versus 16% on placebo (p<0.0001).
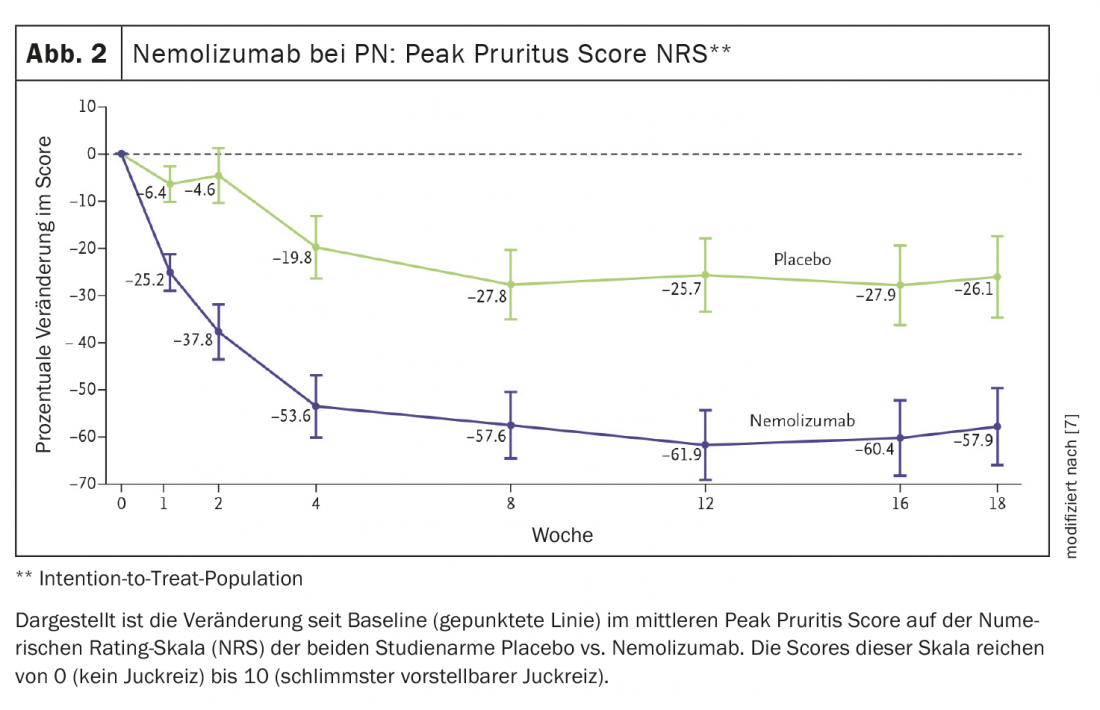
Nemolizumab is also a promising drug candidate. IL-31 is a driver of inflammatory processes and is considered a master cytokine for pruritus. In studies, nemolizumab successfully controlled pruritus in patients with atopic dermatitis and also reduced itch-related sleep disturbances [6]. With this in mind, a phase II clinical trial of nemolizumab in PN was conducted. Results published in the New England Journal of Medicine showed that nemolizumab significantly reduced pruritus in patients with moderate to severe prurigo nodularis [7]. Seventy patients were randomized 1:1 to nemolizumab or placebo, and the initial pruritus score according to the NRS was 8.4. At week 4, peak pruritus NRS decreased by 4.5 points (-53.0%) in nemolizumab-treated patients compared with baseline, but by only 1.7 points (-20.2%) in the placebo group (p<0.001) [7] (Fig. 2).
The FDA has already approved nemolizumab and dupilumab for the indication PN, and Prof. Wollenberg hopes that Europe will follow suit in the near future.
We can also look forward to the JAK inhibitors
In addition to these two biologics, there are also a number of small molecule drugs that are believed to have therapeutic potential in chronic prurigo. Delgocitinib is a topical JAK inhibitor that inhibits JAK-1, JAK-2, and JAK-3 and may also be effective in CPG. In Japan, delgocitinib has been approved for the treatment of atopic dermatitis [8]. Another JAK inhibitor in topical dosage form is ruxolitinib. This inhibits JAK-1, JAK-2, and TYK-2. Ruxolitinib is approved in the United States for the indication of atopic dermatitis [9]. Tofacitinib is an oral JAK inhibitor that inhibits JAK-3, JAK-2, and TYK-2 and has been shown to be effective in treating PN in a case report [10].
Also, the use of the neuroactive substance aprepitant resulted in a significant decrease in the intensity of itch in PN (p<0.05). [11]. However, aprepipant is a relatively costly treatment option that has not yet gained acceptance in routine care, the speaker said. In a case series, the combination of MTX and alitretinoin proved effective for the treatment of PN [12]. Regarding the conventional systemic agents MTX, ciclosporin A, and azathioprine, the expert advises caution because CPG patients are often elderly and, in particular, relatively many of the PN patients have impaired renal function.
Congress: SGDV Annual Congress
Literature:
- Pereira MP, et al: EADV Task Force Pruritus group members. European academy of dermatology and venereology European prurigo project: expert consensus on the definition, classification and terminology of chronic prurigo. J Eur Acad Dermatol Venereol 2018; 32(7): 1059-1065.
- “Chronic prurigo – state of the art in your practice”, Prof. Dr. med. Dr. h.c.. Andreas Wollenberg, SGDV Annual Meeting 09.11.2022.
- Lönndahl L, et al. Dupilumab significantly reduces symptoms of prurigo nodularis and depression: a case series. Acta Derm Venereol 2022; 102 : adv00754XX.
- Study of dupilumab for the treatment of patients with prurigo nodularis, inadequately controlled on topical prescription therapies or when those therapies are not advisable (PRIME2). Available from: https://ClinicalTrials.gov/show/NCT04202679. Accessed April 11, 2022.
- “Dupixent® (dupilumab) is the first biologic to significantly reduce itch and skin lesions in phase 3 trial for prurigo nodularis, demonstrating the role of type 2 inflammation in this disease,” Sanofi, October 22, 2021, www.sanofi.com/en/media-room/press-releases/2021/2021-10-22-05-00-00-231…,(last accessed 11/15/2022)
- Ruzicka T, et al: NEJM 2017 ; 376 : 826-835.
- Stand S, et al: NEJM 2020; 382: 706-716.
- Worm M, et al: Br J Dermatol 2022; 187: 42-51.
- Papp K, et al: JAAD 2021; 85: 863-872.
- Peng C, et al: Clin Cosmet Investig Dermatol 2022 ; 15: 503-506.
- Angelopoulos K, et al. JEADV 2019 ; 33 : 2371-2379.
- Bergqvist C, et al: JEADV 2021; 35: e516-e519.
- Paller AS, et al: JAAD 2005; 52 : 810-822.