About 7% of the population suffers from a rare disease, in Switzerland. Many of the more than 7000 known orphan diseases are in the oncology field, and more than 40% of the drugs developed for rare diseases are used to treat cancer. However, it is not only the increased market introduction of so-called orphan drugs that benefits patients, but also the general shift in cancer medicine towards personalized treatments and immunotherapy.
An orphan disease is any condition that occurs in no more than five out of 10,000 people. Between 6000 and 8000 such conditions are known, and new ones are added daily [1]. Often, these are little-known conditions, which can mean that sufferers already have a long way to go before the correct diagnosis is finally made, let alone adequate treatment is initiated. In many cases, this does not even exist, as research and development in the field of orphan drugs is extremely difficult due to low case numbers and is usually not worthwhile for the manufacturers without corresponding financial support [3].
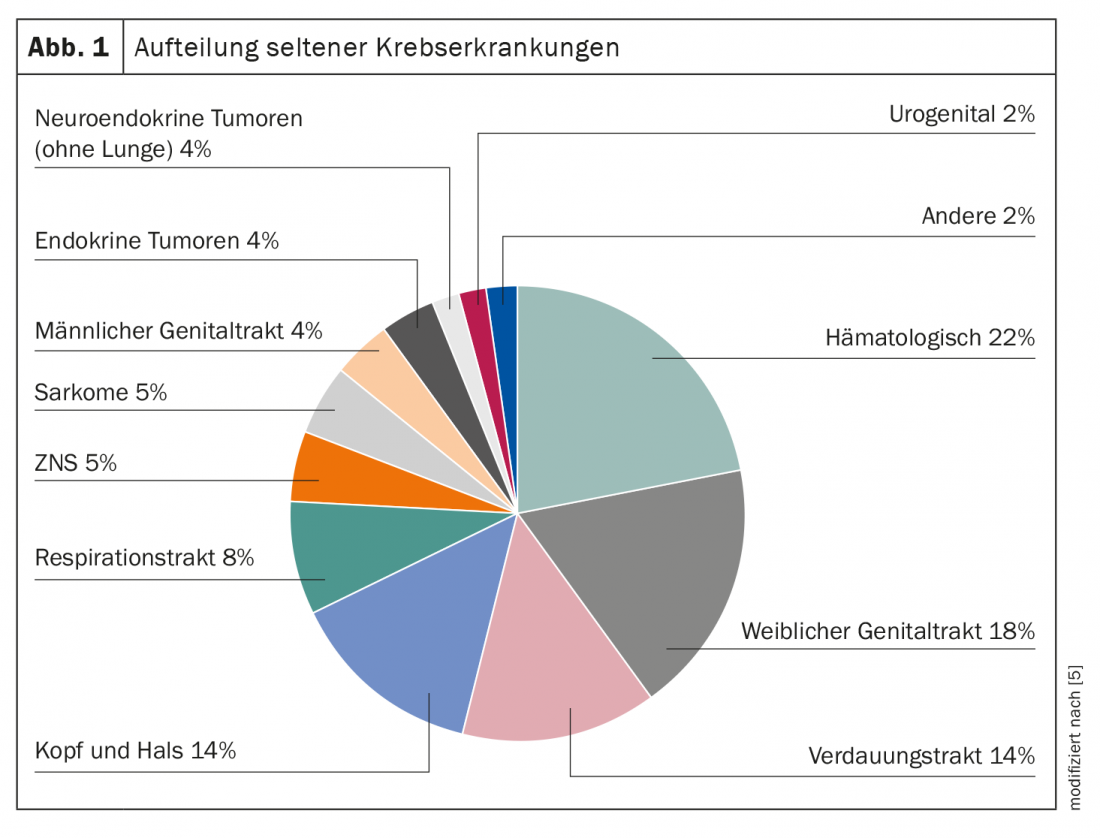
Rare diseases are not uncommon, especially in metabolic medicine and oncology. For example, orphan diseases account for approximately 20% of all cancers [4]. These include urogenital, neuroendocrine, hematologic, and many pediatric conditions, as well as malignancies from numerous other areas (Fig. 1) [5]. Mast cell leukemia, olfactory neuroblastoma, malignant insulinoma, and synovial sarcoma are just a few examples from the list of several hundred entities [6]. As different as the individual orphan diseases may be, the challenges faced by sufferers and caregivers after diagnosis are nevertheless similar.
Treatment outside the grid
Already the correct recognition of the disease is often a big hurdle. However, even when this is overcome, the management of patients with rare cancers often becomes a problem due to the lack of applicable treatment standards and effective medications. In addition to the difficulty of finding enough participants for registration studies, the extremely limited sales market is not exactly conducive to the pharmaceutical industry’s interest in investing resources in the development of orphan drugs. However, Swissmedic’s incentives for research into substances for the treatment of rare diseases, which have increased in recent years, are showing noticeable success. For example, 203 compounds with orphan drug statuswere already approved in Switzerland in 2018, compared to 51 ten years earlier [7]. The measures taken by the Medicines and Medical Devices Agency, which include financial relief, an adapted approval process and extended document protection – that is, the protection of documents submitted as part of the approval process – of 15 years, are certainly an important and effective step [8,9]. Nevertheless, there are still far too few proven drugs for the treatment of rare diseases today.
In addition to the slow development of orphan drugs, their reimbursement is often fraught with difficulties. The complex research in combination with the small number of customers often results in horrendous prices, which the health insurance companies are not willing and able to pay. Only if a new drug makes it onto the federal government’s list of specialties does the cost have to be reimbursed by the health insurer [9]. In addition to effectiveness and expediency, this also requires economic efficiency. In addition, by no means all substances used to treat rare diseases were developed specifically for this purpose. Frequently, drugs are administered off-label for lack of better options [4]. Here, too, funding can quickly become a problem, as each case must be assessed individually and proof of efficacy and cost-effectiveness must be provided [10]. Overall, sufferers are faced with the unsatisfactory situation that not only are there fewer therapeutic options for their condition, but that these are also more difficult to access. So apart from scientists and pharmaceutical companies, regulatory authorities and policy makers are also challenged when it comes to improving care in the field of orphan diseases.
All these considerations raise an unpleasant and burning question: How expensive can a drug be in relation to its effect? Or to put it another way: Is distributive justice guaranteed? This issue repeatedly leads to conflicts between authorities and manufacturers, as a consequence of which substances are not available for months or even years after their approval [7]. A rope pull on the backs of patients that will probably reach its sad climax while the price spiral continues to spiral upward.
Opportunities through new order and cross-tumor approaches.
With the development of targeted therapies and the increasing genetic classification of tumor diseases, independent of the previously widespread site-specific classifications, new horizons are also emerging in the therapy of orphan diseases. For example, larotrectinib for the treatment of solid tumors with NTRK fusion proteins was the first substance with an entity-independent indication to be approved in Switzerland [11]. This approach could increase the availability of adequate treatment options in the future, regardless of whether or not it is an orphan drug , as is the case with larotrectinib.
Immunotherapy, which in its basic features has potential for treating all types of cancer, is also an important source of hope for many people affected by rare diseases. If valid predictive markers can be identified that have tumor-independent validity, good patient selection would be possible and broader application in rare tumors would be a logical consequence. Underpinning this approach is the approval of pembrolizumab in the US, also for tumor diagnosis [12].
Whether personalized medicine or immunotherapy, genetic classification and predictive markers are likely to shape the future of rare cancer management. It is essential for their further research that they are recorded as completely as possible, especially with regard to their genetic profile. Some approaches to this exist in Switzerland, such as the Onconavigator, but they are still in their infancy [13].
The power of cumulative frequency
Since sufficient patient numbers play an essential role for the development of therapeutic standards and treatment methods, as well as for the robust characterization of disease patterns, the research and care of orphan diseases must not take place behind closed curtains. Collaboration across national borders is essential, especially for small countries like Switzerland. However, pooling knowledge and energy makes sense not only in research and development, but also in the care of those affected. Only with successful coordination can patients be cared for at centers where the necessary expertise to treat their disease is available. Corresponding efforts and developments in recent years, both in the field of pharmacology and policy and in the establishment of networks and self-help groups, are probably largely due to the cumulative frequency of rare diseases. We now need to take advantage of this not only in terms of the entity collection pot, but also geographically.
Literature:
- Federal Office of Public Health FOPH: Rare Diseases. 25.04.2019. www.bag.admin.ch/bag/en/home/krankheiten/krankheiten-im-ueberblick/viele-seltene-krankheiten.html (last accessed 28.10.2020)
- diabetesschweiz: About diabetes. www.diabetesschweiz.ch/ueber-diabetes.html (last accessed 28.10.2020)
- www.pharma-fakten.de: Orphan drugs: 44 percent of developments are directed against different types of cancer. 24.02.2017. www.pharma-fakten.de/fakten-hintergruende/newsbites/pharma-fakten-grafik-orphan-drugs-44-prozent-der-entwicklungen-richten-sich-gegen-unterschiedliche-krebsarten/ (last accessed 28.10.2020)
- Nitz P: Rare cancers – stepchild of cancer research? 26.01.2017. www.krebsgesellschaft.de/onko-internetportal/basis-informationen-krebs/basis-informationen-krebs-allgemeine-informationen/seltene-krebsarten.html (last accessed 28.10.2020)
- Rare Cancers Europe: “FAMILIES” AND LIST OF RARE CANCERS. www.rarecancerseurope.org/About-Rare-Cancers/families-and-list-of-rare-cancers (last accessed 28.10.2020)
- Surveillance of Rare Cancers in Europe RARECARE: RARECARE Cancer List. www.rarecare.eu/rarecancers/rarecancers.asp (last accessed 28.10.2020)
- Proraris: orphan drugs. www.proraris.ch/de/arzneimittel-seltene-krankheiten-24.html (last accessed 28.10.2020)
- www.swissmedic.ch (last accessed 28.10.2020)
- Werder C: Approval of orphan drugs in Switzerland. 13.05.2017. www.sma-schweiz.ch/wp-content/uploads/2017/07/SMA-Schweiz-Tag-2017_Swissmedic.pdf (last accessed 28.10.2020)
- Swiss Society of Medical Officers and Insurance Physicians: Manual Oncology. www.vertrauensaerzte.ch/manual/chapter34.html (last accessed 28.10.2020)
- swissmedic Drug Information. www.swissmedicinfo.ch/ (last accessed 28.10.2020)
- FDA: FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication.www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication (last accessed Oct. 28, 2020)
- Ackermann P: “Only sharing gets us further”. Swiss Cancer Bulletin 2/2020: 108-111.
InFo ONCOLOGY & HEMATOLOGY 2020; 8(6): 18-19.