The identification and study of molecular biomarkers may help to dissect the complex phenotype of atopic dermatitis into subphenotypes. A long-term goal of this translational research is the stratification and selection of patients on the basis of diagnostic, prognostic and predictive biomarkers as a basis for an individually optimally suited therapy strategy.
Numerous recent publications address the topic of translational research approaches in the context of atopic dermatitis (AD). Among them, a review paper published in Cellular & Molecular Immunology 2023 by Facheris et al. and a review by Balato et al. published in Life 2022 [1,2]. The following is a summary of some of the key findings addressed therein [1,2].
Translational research for personalized therapy approaches
Recent advances in the understanding of AD pathogenesis have revolutionized translational research and led to an exponential expansion of the therapeutic pipeline [1]. In addition to the already approved biologics (Dupilumab, Tralokinumab) and JAK inhibitors (Baricitinib, Upadacitinib, Abrocitinib), numerous other innovative active substances such as biologics and “small molecules” are currently in development for the indication area of atopic dermatitis. These include lebrikizumab (anti-IL13), nemolizumab (anti-31-R), tezepelumab (anti-TSLP), anti-Ox-40-Ak as well as brepocitinib (topical TYK2/JAK1 inhibitor) and aryl hydrocarbon receptor modulators [1,2]. Based on the assumption that heterogeneous symptom expression and disease progression reflect fundamental differences at the molecular level, it should be possible in the future to identify patient subgroups with different subtypes of the disease and different responses to specific therapies.
| Translational research projects In the project “BIOMAP “* (Biomarkers in Atopic Dermatitis and Psoriasis) [14] causes and mechanisms of AD and psoriasis are investigated with the aim to identify biomarkers responsible for variation in disease progression. The focus of the project “CHAMP”$ (CHildhood Allergy and tolerance: bioMarkers and Predictors) [15] is to investigate determinants of various allergic diseases (food allergy, atopic dermatitis, asthma, hay fever) from birth to adolescence. Special attention is paid to factors determining primary tolerance (=no occurrence of diseases) and acquired tolerance (=remission of existing diseases). The study project will identify clinically relevant biomarkers that can be used to predict early onset, progression and remission. |
* Duration: 01.04.2019-31.03.2024 $ Duration: 2017-2022 |
AD: multifactorial pathophysiology with genetic predisposition.
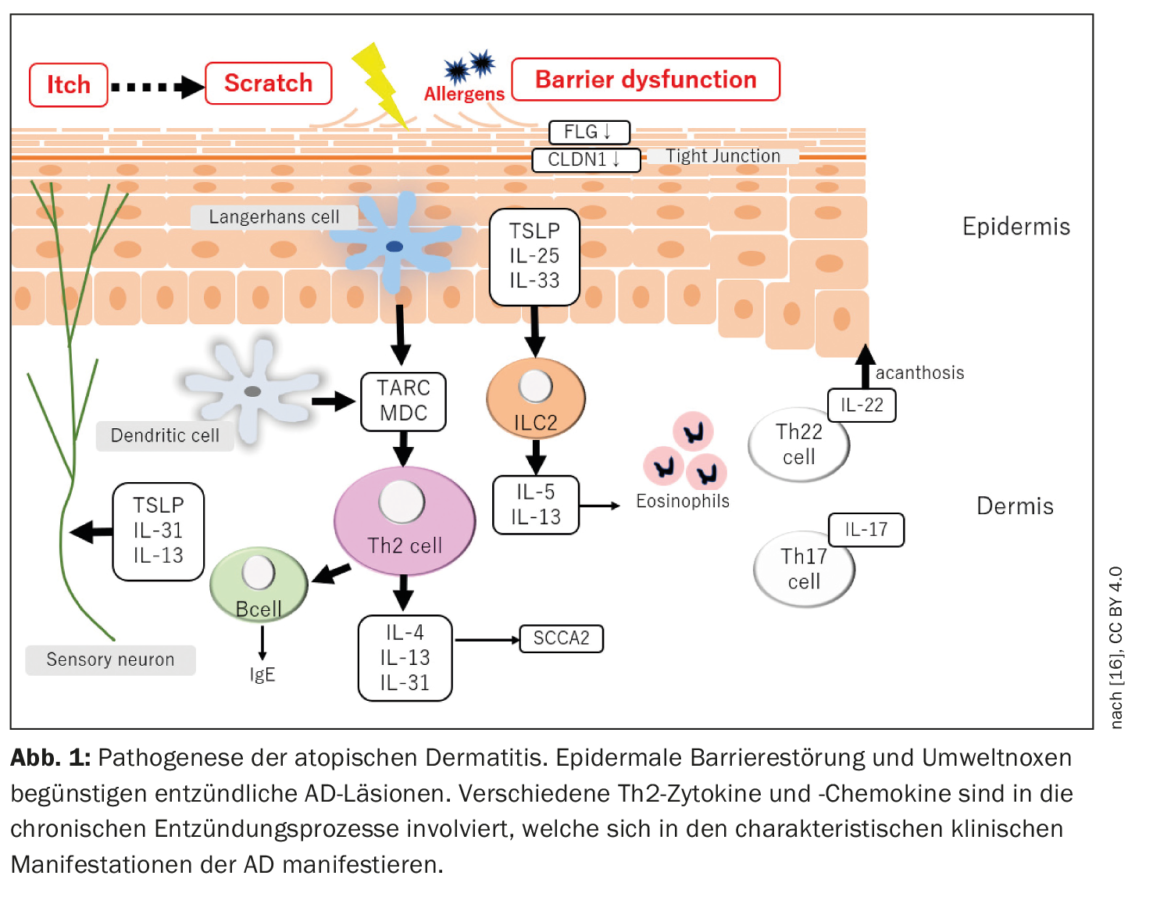
Atopic dermatitis is characterized by a relapsing chronic recurrent course with onset of disease in early childhood. Acute “flares” are accompanied by inflammatory skin lesions and severe itching, so that the patients’ quality of life is considerably impaired [3]. The pathophysiology of AD is complex (Fig. 1) : Interactions between impaired skin barrier function and modified immune responses contribute to the development and maintenance of the disease [2].
It is known that a genetic predisposition is a prerequisite for the development of AD. The best known genetic risk factor is a mutation in the FLG gene, which leads to reduced filaggrin expression in the skin and associated epidermal barrier disruption [4]. In addition to FLG, more than 30 genetic loci have been identified to be associated with AD [5,6]. In addition, variants in the IL-13 gene or IL-6 receptor region, as well as several rare protein-coding variants, explain nearly 30% of the genetic susceptibility to AD [7]. AD is considered a Th2-mediated disease, which is supported by findings of increased levels of Th2 mediators and decreased levels of IFN-γ in the blood of patients with severe AD [1,8]. Several studies confirmed that AD lesions are primarily, but not exclusively, Th2-mediated, and are associated with overproduction of important Th2 cytokines and chemokines. However, Th22 also seems to be involved, as reflected by an overproduction of IL-22, while the involvement of the Th1 and Th17 axes apparently varies depending on the AD endophenotype [1].
Identification of clinically relevant biomarkers remains a challenge
Projects investigating clinically relevant biomarkers take advantage of the latest technical developments in translational medicine with the long-term goal of improving treatment options and “disease management”.
A criterion for molecular biomarkers is that they are detected and measured in blood, other body fluids, or tissue and can be used as indicators of disease-related processes in the body [9].
Currently, at least 18 blood/serum detectable biomarkers are known to be associated with AD disease activity, such as levels of eosinophil granulocytes, lactate dehydrogenase, total IgE, or soluble IL-2 receptor [10]. In addition, several chemokines# (CCL17, CCL18, CCL22, CCL26, CCL27) and interleukins (IL-13, IL-22, IL-24, IL-25, IL-31, IL-33), as well as “Thymic Stromal Lymphopoietint” (TSLP), periostin, and squamous cell carcinoma antigen-2 are considered biomarkers in AD [10]. CCL18, TSLP and CCL26 were associated with comorbid asthma in a Danish study in children suffering from AD [11]. The researchers studied four groups of 6-12-year-olds: (1) past or current AD without asthma, (2) past or current AD and current asthma, (3) current asthma without AD. (4) Healthy controls. An exploratory clinical trial was conducted to investigate which biomarkers are associated with treatment response to IL-4 receptor-α inhibition by the monoclonal antibody dupilumab in moderate-to-severe AD. Dupilumab was shown to reduce the increased expression of Th2 signatures such as IL-13, IL-31, CCL17, CCL18, CCL22, and CCL26 in the blood and lesional skin of AD [12,13]. Balato et al. indicate that further studies are needed to find out more about possible associations of these biomarkers with disease and treatment outcome in AD [2].
# Chemokines are a subgroup of cytokines; they are small (8-10 kDa), chemotactically active proteins (signal proteins).
Literature:
- Facheris P, et al: The translational revolution in atopic dermatitis: the paradigm shift from pathogenesis to treatment. Cell Mol Immunol 2023,
https://doi.org/10.1038/s41423-023-00992-4,(last accessed Feb. 24, 2023). - Balato A, et al: The Impact of Psoriasis and Atopic Dermatitis on Quality of Life: A Literature Research on Biomarkers. Life. 2022; 12(12): 2026.
- Arima K, et al: Burden of atopic dermatitis in Japanese adults: analysis of data from the 2013 National Health and Wellness Survey. J Dermatol 2018; 45, 390-396.
- Irvine AD, et al: Filaggrin mutations associated with skin and allergic diseases. N Engl J Med 2011; 365: 1315-1327.
- Ellinghaus D, et al: High-density genotyping study identifies four new susceptibility loci for atopic dermatitis. Nat Genet 2013; 45: 808-812.
- Løset M, et al: Genetics of Atopic Dermatitis: From DNA Sequence to Clinical Relevance. Dermatology 2019; 235: 355-364.
- Mucha S, et al: Protein-coding variants contribute to the risk of atopic dermatitis and skin-specific gene expression. J Allergy Clin Immunol 2020, 145, 1208-1218.
- Gittler JK, et al: Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol 2012; 130: 1344-1354.
- European Medicines Agency (EMA). Qualification of Novel Methodologies for Medicine Development. Human Regulatory. Research and Development. Opinions and Letters of Support on the Qualification of Novel Methodologies for Medicine Development. Available online: www.ema.europa.eu/en/glossary/biomarker,(last accessed Mar. 24, 2023).
- Nakahara T, et al: Exploration of biomarkers to predict clinical improvement of atopic dermatitis in patients treated with dupilumab: A study protocol. Medicine 2020; 99: e22043.
- Basu MN, et al: Biomarkers in asthma in the context of atopic dermatitis in young children. Pediatr Allergy Immunol 2022; 33: e13823.
- Guttman-Yassky E, et al: Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis. J Allergy Clin Immunol 2019; 143: 155-172.
- Hamilton JD, et al: Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol 2014; 134: 1293-1300.
- Precision Medicine in Chronic Inflammation, www.precisionmedicine.de/fileadmin/user_upload/cluster/pmi/downloads/PMI-Magazin2021_digital_Doppelseiten.pdf,(last accessed Mar. 24 , 2023).
- BMFB: CHAMP – Allergy and tolerance in childhood: biomarkers and predictors, www.gesundheitsforschung-bmbf.de/de/champ-allergie-und-toleranz-im-kindesalter-biomarker-und-pradiktoren-7268.php,(last accessed 03/24/2023).
- Itamura M, Sawada Y: Involvement of Atopic Dermatitis in the Development of Systemic Inflammatory Diseases. International Journal of Molecular Sciences. 2022; 23(21): 13445, www.mdpi.com/1422-0067/23/21/13445,(last accessed Mar. 23 , 2023).
DERMATOLOGIE PRAXIS 2023; 33(2): 28-29