Nanobiomaterials offer a revolutionary opportunity to improve the treatment of cardiac arrhythmias. From targeted drug delivery to tissue regeneration and continuous monitoring, they open up new horizons for personalized medicine. Future advances in materials science, biotechnology and genomics could accelerate the clinical translation of these technologies and help to sustainably improve the quality of life of millions of patients worldwide.
(red) Cardiac arrhythmias are among the most common and potentially life-threatening complications of cardiovascular diseases (CVDs), which are the leading cause of death worldwide. In 2021, CVDs led to more than 20.5 million deaths, and this number is projected to rise to 23.6 million by 2030. Cardiac arrhythmias are caused by abnormalities in the heart’s electrical signal transmission, which disrupts both the frequency and rhythm of heartbeats. This not only impairs the mechanical function of the heart, but also significantly increases the risk of sudden death. From common atrial fibrillation to rare genetic arrhythmia syndromes, these disorders pose an immense medical and social challenge.
Although conventional treatments such as drug therapies, radiofrequency ablation and implantable cardiovascular electronic devices (CIEDs) such as pacemakers and defibrillators can be effective, they are often associated with side effects, limited efficacy and significant risks of invasive procedures. However, recent advances in nanotechnology have ushered in a new era in the treatment of cardiac arrhythmias. Nanobiomaterials, characterized by their small size, high specificity and unique physical and chemical properties, enable targeted and sustainable treatment strategies that can complement or even replace conventional approaches.
Electrophysiology of the heart and the mechanisms of cardiac arrhythmias
The normal function of the heart is based on a precise and coordinated electrical signaling network. This network begins in the sinus node, which acts as the primary pacemaker of the heart and generates the initial electrical impulses. These signals propagate through the atria and are delayed by the atrioventricular node (AV node) before being transmitted to the ventricles via the His bundle and Purkinje fibers. This orderly conduction ensures synchronous contraction of the heart muscles and guarantees efficient pumping of the heart.
Cardiac arrhythmias are caused by disturbances in this electrical system, which can be caused by three main mechanisms: abnormal automaticity, triggered activity and reentry phenomena. Abnormal automaticity describes an increased spontaneous excitation of certain cardiac muscle cells, typically occurring in the sinus node or Purkinje fibers. Triggered activity results from excessive calcium storage in the cells, which can trigger additional impulses outside the normal cardiac cycle. Reentry phenomena are caused by circular excitations that become permanently established in the heart due to structural or electrical changes and can lead to arrhythmias such as atrial fibrillation.
The treatment of these complex mechanisms requires highly specialized approaches that go beyond current standards. Nanobiomaterials offer unique opportunities for targeted intervention at the molecular and cellular level.
Nanobiomaterials as innovative therapeutic approaches
Traditional drug treatment of cardiac arrhythmias is often limited by low bioavailability, non-specific effects and serious side effects. Nanobiomaterials can overcome these challenges through their ability to deliver drugs to specific target tissues. Nanoparticles enable controlled release of drugs, which can reduce the dose and minimize side effects.
One example is amiodarone, a class III antiarrhythmic drug that effectively treats arrhythmias but can damage the liver and lungs due to its systemic toxicity. Amiodarone embedded in nanoparticles can ensure slow and targeted release, reducing toxic effects on unaffected tissues. Similarly, nanoparticles loaded with carvedilol can improve the bioavailability of this antiarrhythmic drug while minimizing its side effects.
Another promising example is the use of hydrogels that are temperature- or pH-sensitive and release drugs such as budesonide into inflamed tissue. This is particularly beneficial after radiofrequency ablation, as it can reduce the recurrence of atrial fibrillation caused by inflammation.
Cardiac tissue engineering: regeneration and repair
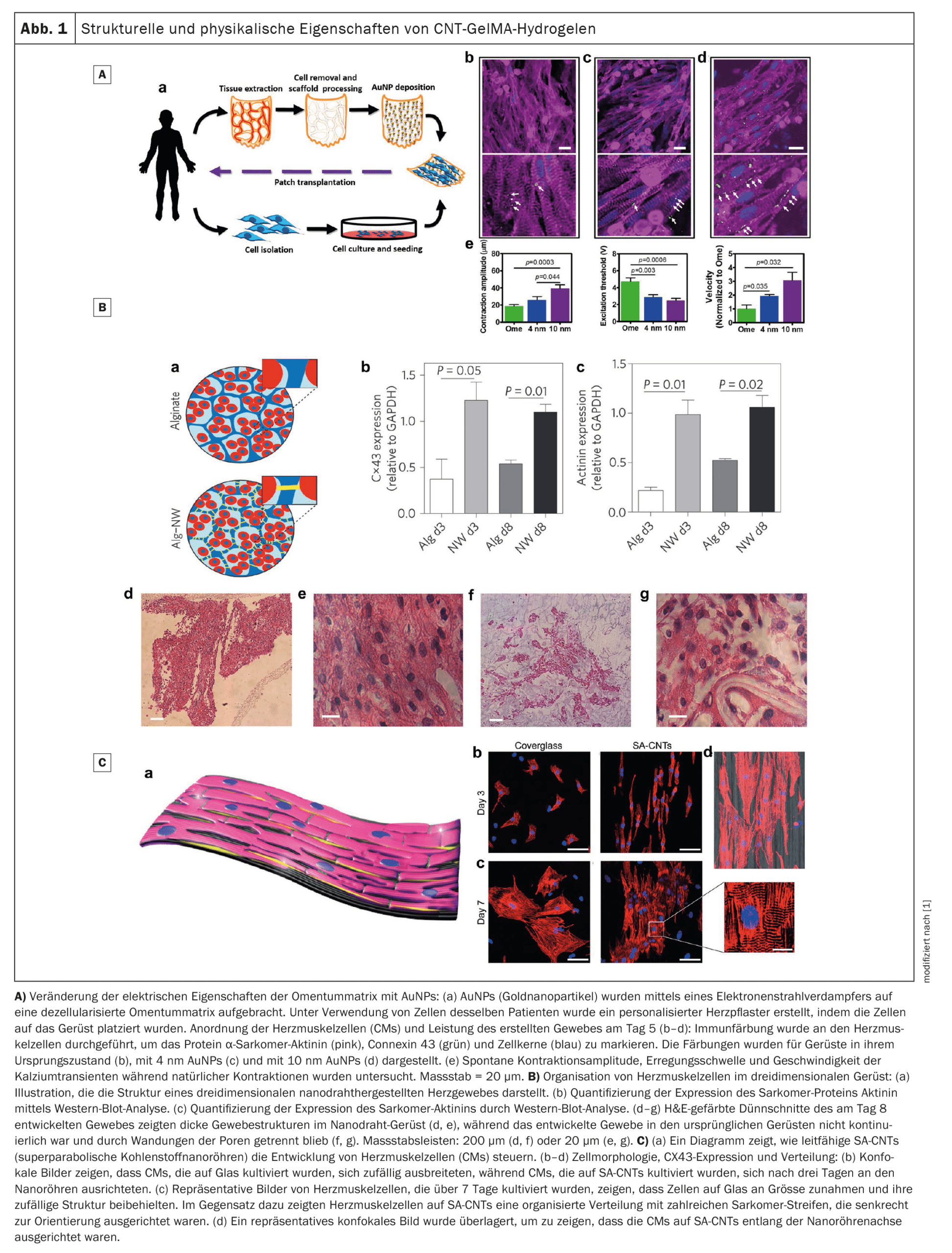
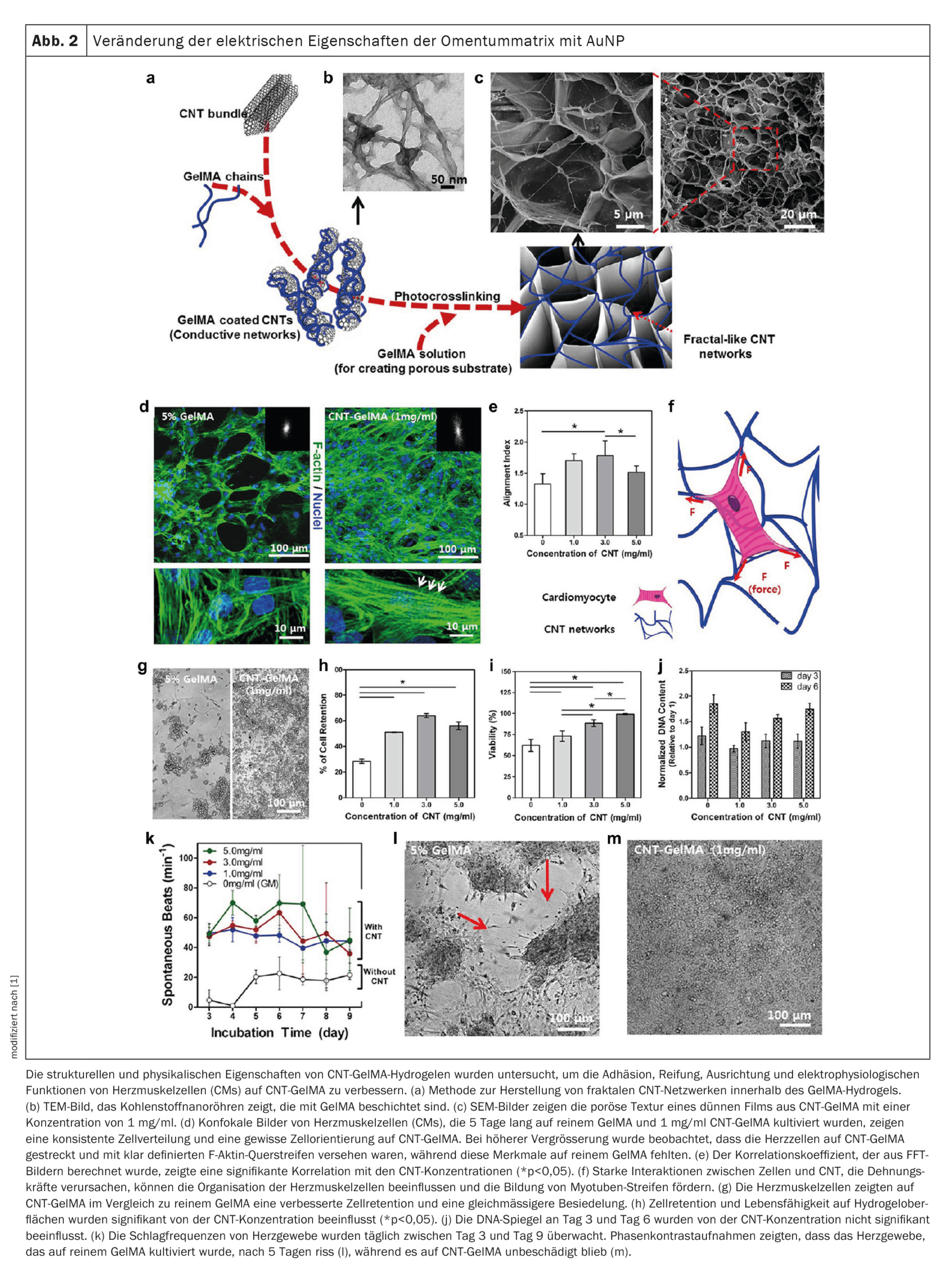
The regeneration of the myocardium after a heart attack is severely restricted due to the limited ability of cardiomyocytes to divide. Nanobiomaterials integrated into scaffold structures offer innovative solutions here. These scaffolds can serve as platforms for cell adhesion and promote the growth of heart muscle cells. Nanostructures such as carbon nanotubes (CNTs) or graphene can improve the electrical conductivity of such scaffolds, which facilitates the synchronization of contractions in damaged tissue.
A notable example is the development of hybrid hydrogel scaffolds that combine CNTs and gelatin methacrylate. These scaffolds promote electrical coupling between cells, increase contractility and improve cell maturity. In addition, such scaffolds can be further optimized by integrating gold nanowires or graphene to improve conductivity and mechanical stability.
Cardiac patches based on alginates or polypyrrole also offer promising approaches. These patches can efficiently transmit electrical signals, which restores the functionality of the damaged myocardium. In addition, studies have shown that the combination of such patches with nanomaterials such as graphene or gold nanoparticles promotes the expression of connexin 43, a protein that is crucial for electrical signal transmission between heart muscle cells.
Biosensors: Early diagnostics and monitoring
Nanobiomaterials have enabled the development of highly sensitive biosensors that can detect specific cardiac markers such as brain natriuretic peptide (BNP). Such sensors can be integrated into wearable devices to continuously monitor heart function and detect arrhythmias at an early stage.
One notable example is a biosensor based on a reduced graphene oxide platform modified with platinum nanoparticles. This sensor can detect BNP concentrations in the femtomolar range and offers higher sensitivity than conventional methods such as ELISA. Integrated nanocomposites in heart patches also enable real-time monitoring of electrical signals and targeted stimulation, opening up new possibilities for the treatment of cardiac arrhythmias.
Challenges and limitations
Despite their promising properties, nanobiomaterials face several challenges. The potential toxicity and immunogenicity of these materials require extensive preclinical and clinical studies. In addition, the large-scale production of such materials is costly and complex, which may hinder their widespread clinical application. The development of standardized manufacturing processes and safe degradation products remains a priority for future integration into medical practice.
Nanobiomaterials offer a revolutionary opportunity to improve the treatment of cardiac arrhythmias. From targeted drug delivery to tissue regeneration and continuous monitoring, they open up new horizons for personalized medicine. Future advances in materials science, biotechnology and genomics could accelerate the clinical translation of these technologies and help to sustainably improve the quality of life of millions of patients worldwide.
Source:
- Lu D, Fan X: Insights into the prospects of nanobiomaterials in the treatment of cardiac arrhythmia. J Nanobiotechnology. 2024 Aug 30;22(1): 523. doi: 10.1186/s12951-024-02805-w. PMID: 39215361; PMCID: PMC11363662.
CARDIOVASC 2024; 23(4): 37-40