Anxiety and depressive disorders have overlapping symptoms and common neurobiological pathways. Anxiety disorders have also been shown to predict later depression and vice versa. Therefore, it is not surprising that antidepressants are effective and recommended for anxiety, just as anti-anxiety medication is recommended for depression. The efficacy of the anti-anxiety medication Silexan has now been the subject of a study on major depressive disorder (MDD).
Silexan is an essential oil for oral administration obtained from the flowers of Lavandula angustifolia and is registered as a medicinal product for patients with anxiety disorders. The efficacy of the herbal active ingredient in subthreshold and generalized anxiety disorders as well as in mixed anxiety and depression disorders (MADD) has been demonstrated in randomized, double-blind, placebo-controlled and referenced clinical trials and has already been the subject of several reviews.
The antidepressant effect of Silexan can be explained by its pharmacological profile. Silexan has been shown to promote several parameters of neuroplasticity in cell and in vivo models. In one study, an upregulation of the neuronal marker protein GAP-43 and a significant increase in neurites was observed under the influence of Silexan. A positive effect on synaptogenesis was observed even at low Silexan concentrations. Another study showed that the inhalation of commercially available lavender oil increases neuronal proliferation and dendritic complexity in the hippocampus and supraventricular zone and raises serum levels of the neurotrophic factor BDNF.
These results suggest a benefit of lavender oil on neuroplasticity and neurogenesis, which seem to be the common pathway for antidepressants in general. Silexan causes a significant increase in the density of 5-HT1A receptors and decreases their binding potential, leading to an increase in extracellular serotonin, dopamine and noradrenaline, a mechanism normally observed with serotonergic antidepressants. In addition, lavender oil has been shown to reduce the increase in immobility times caused by corticosterone, thereby improving depression-like behavior. Following a series of clinical trials investigating the effects of Silexan on depression secondary to or associated with anxiety, Dr. Siegfried Kasper from the Center for Brain Research at the Medical University of Vienna and colleagues from Germany and Switzerland conducted the first randomized, placebo-controlled trial on the efficacy of Silexan in major depressive disorder (MDD) [1].
Endpoint Change in total MADRS value
Patients with a mild or moderate, single or recurrent episode of major depression and a total score of 19-34 points on the Montgomery Åsberg Depression Rating Scale (MADRS) were randomized to receive 1× 80 mg/d Silexan, 1× 50 mg/d sertraline or placebo for 56 days in a double-blind and double-blind dummy procedure.
Participants first underwent a 3-7 day treatment-free screening phase, after which eligible subjects were assigned in a 1:1:1 ratio (baseline visit) to double-blind treatment with Silexan, sertraline or placebo. After 56 days of randomized treatment, a seven-day follow-up phase was conducted to reduce the dose of sertraline to minimize the risk of SSRI discontinuation symptoms, with subjects from the other groups receiving placebo. Treatment efficacy and safety were assessed in participants 1, 2, 4, 6 and 8 weeks after the first visit and safety at the end of the follow-up.
Immediate-release soft capsules containing 80 mg Silexan were used. Film-coated tablets containing 50 mg sertraline were obtained commercially and packaged in capsules. Identical placebo capsules were available for Silexan and sertraline. The odor of the study drugs was matched by flavoring the Silexan placebo capsules with 1/1000 of the amount of lavender oil contained in the Silexan capsules. The primary endpoint was the change in total MADRS score between baseline and end of treatment.
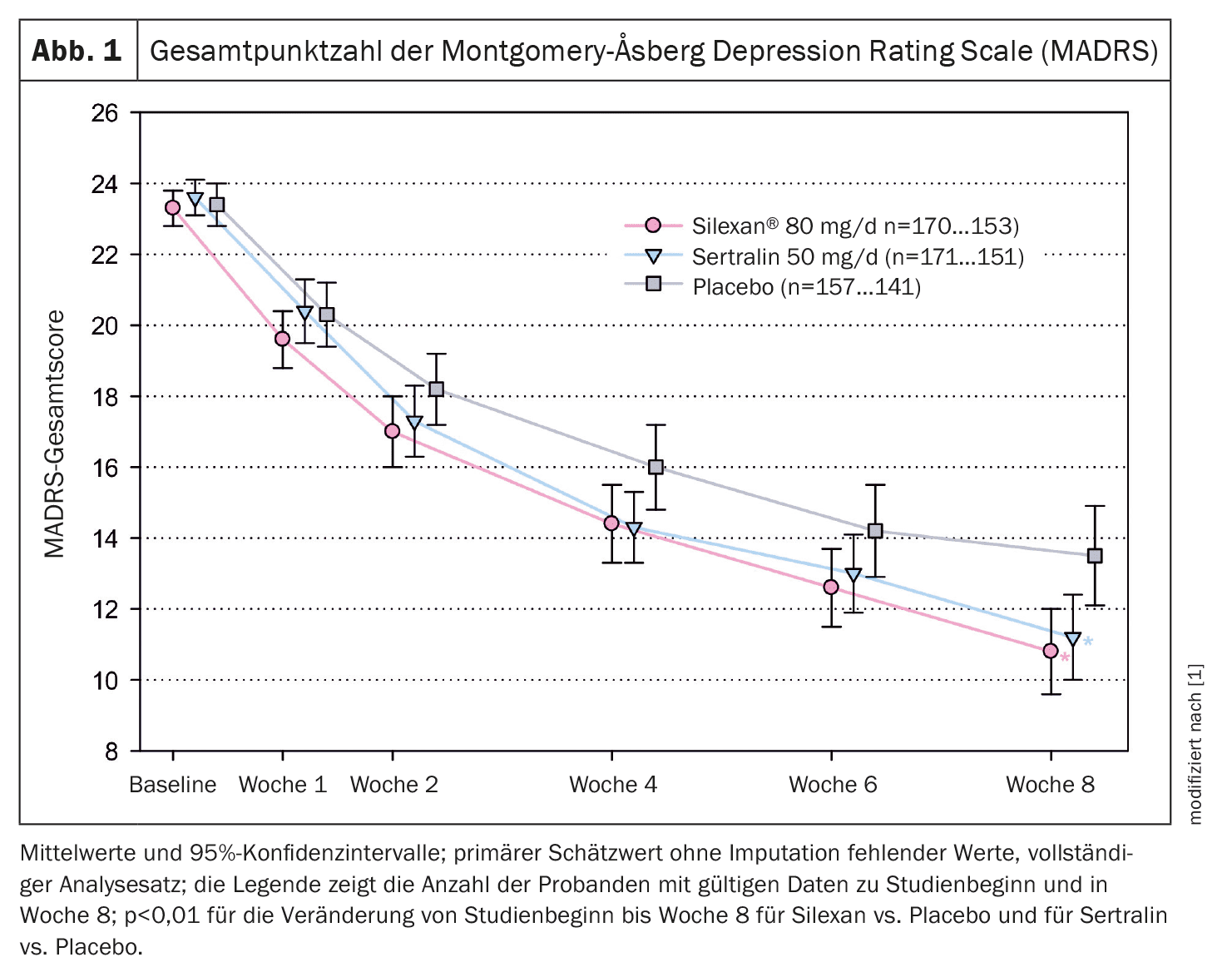
The study was conducted between October 2020 and April 2023 in 53 general medical, psychiatric and neurological practices in Germany and Poland. A total of 498 patients were treated and analyzed (Silexan 170, sertraline 171, placebo 157). The mean total MADRS score decreased monotonically in all treatment groups until the end of the efficacy assessments at week 8 (Fig. 1). The overall decline in scores in the groups receiving Silexan and sertraline was similar and more pronounced than in the placebo group. Based on the mean intraindividual differences from baseline, Silexan was significantly superior to placebo after the eight-week treatment with a lead of 2.17 points (p<0.01). A significant superiority was also observed for sertraline compared to placebo, which confirms the assay sensitivity of the study.
Silexan superior in the alleviation of functional disorders
The results of the Sheehan Disability Scale (SDS) showed a significant improvement in functional impairment associated with depression in patients treated with Silexan compared to the placebo group after 8 weeks, with a difference of 2.40 (1.04-3.76) points.
In addition to the overall score, significant differences were also observed between Silexan and placebo in the individual areas of SDS (work, social life/leisure activities, family life/responsibility for the household) (p≤0.01). No significant improvement in SDS was observed with sertraline compared to placebo.
Both treatments were well tolerated. The most common potentially related events in the Silexan group were regurgitation (28 subjects, 16.5%) and nasopharyngitis (6, 3.5%). For sertraline, the most common events were headache (11, 6.4%), nausea (8, 4.7%) and diarrhea (7, 4.1%). All other potentially dosing-related events were observed in less than 3% of subjects in the silexane or sertraline groups.
According to Dr. Kasper and colleagues, their results show that eight weeks of treatment with Silexan at a dose of 1 × 80 mg/day had a statistically significant and clinically relevant antidepressant effect in people with a mild to moderate, single or recurrent episode of MDD. The observed mean reduction in MADRS total score from baseline (12.1 points at 8 weeks) and a minimum expected mean improvement in the underlying patient population of 11 points (corresponding to the lower limit of the 95% confidence interval for the point estimate) were within the range of clinically meaningful or clinically substantial change according to empirically derived criteria. When comparing Silexan and placebo, the observed mean difference of 2.17 points for the overall change in the MADRS score was above the two-point range considered clinically significant. With a mean difference of 2.59 points compared to placebo, sertraline also showed a clear, statistically significant antidepressant effect.
The authors also note that significant improvements were observed in all functional areas of SDS (work, social life/leisure activities, family life/domestic responsibilities), suggesting that treatment with Silexan had a significant positive effect on disease-related quality of life. This is of particular importance as depression is generally recognized as the main cause of reduced quality of life and work disability. No comparable improvement in aspects of daily life was observed with sertraline, although both therapies had a similar antidepressant effect.
Literature:
- Kasper S, et al: Lavender oil preparation Silexan is effective in mild-to-moderate major depression: a randomized, placebo- and reference-controlled trial. Eur Arch Psychiatry Clin Neurosci 2024; doi: 10.1007/s00406-024-01783-2.
FAMILY PHYSICIAN PRACTICE 2024; 19(12): 46-47