In the non-interventional PIONEER REAL Switzerland study, adult type 2 diabetics treated with oral semaglutide as part of routine clinical care achieved clinically significant improvement in glycemic control with no new safety signals. The results were presented at the SGED Annual Meeting and underpin the promising results of the clinical approval studies.
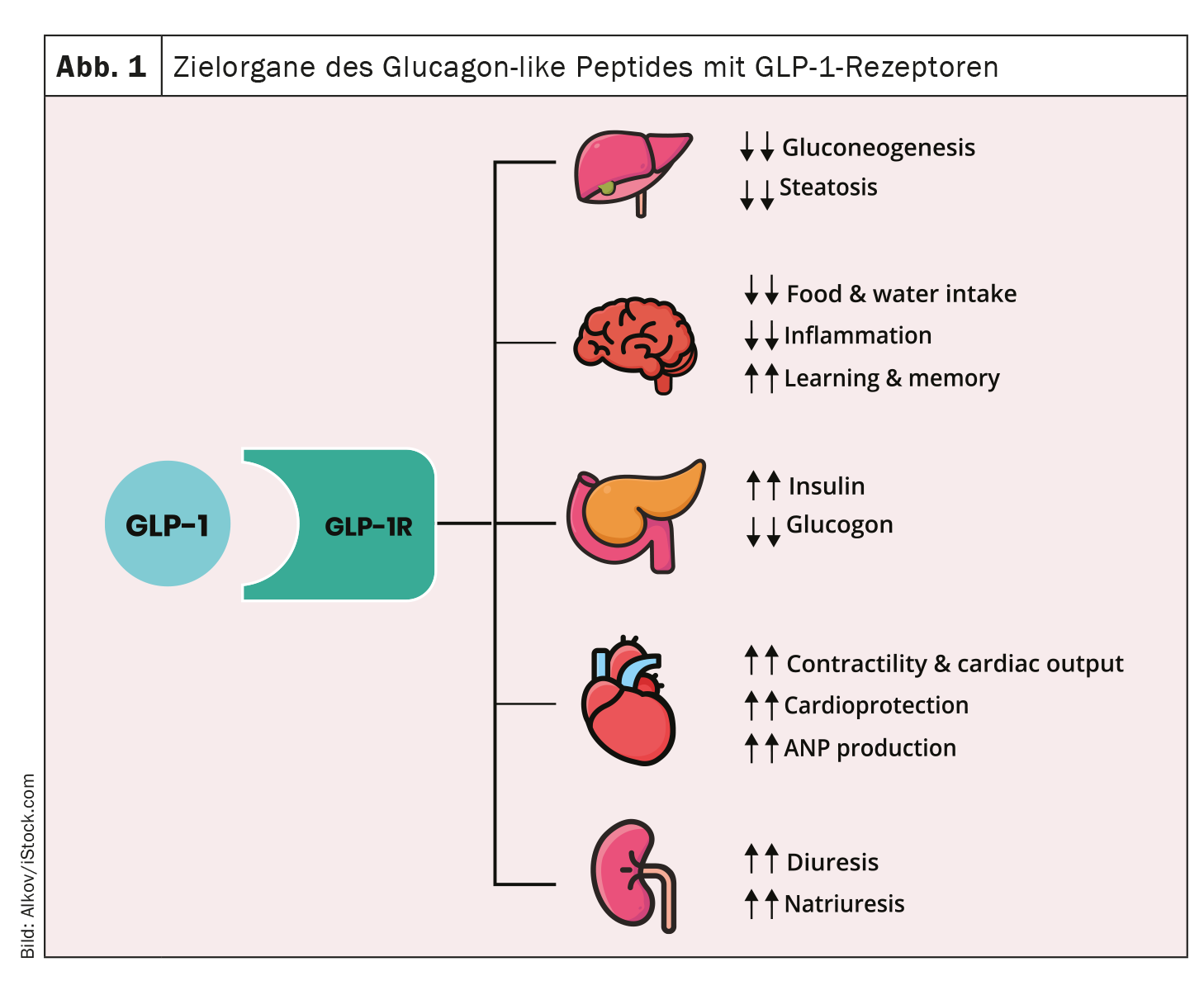
Semaglutide has also been on the market in Switzerland for the treatment of type 2 diabetes (T2D) in oral form since 2020 – under the trade name Rybelsus® [1]. Like other glucagon-like peptide-1 receptor agonists (GLP-1-RA), semaglutide binds to the GLP-1 receptor, thereby promoting insulin secretion and inhibiting glucagon secretion. The Phase III PIONEER trial program was very successful. One of the main studies showed that Rybelsus® not only improves metabolic control and reduces body weight, but also results in fewer heart attacks and even fewer deaths[1,2]. The aim of the international Phase IV study PIONEER REAL is to systematically observe how the drug works and is tolerated under everyday conditions [2].
Multicenter real-world study
PIONEER REAL Switzerland is part of a study project conducted in 13 countries – it is a 34-44-week single-arm, prospective open-label multicenter study in adults with T2D. Participants were treatment-naive to glucose-lowering injection therapies at baseline and received semaglutide in oral form once daily as part of their usual routine care [3]. In addition to changes in HbA1c (primary endpoint) and body weight (secondary endpoint), the proportion of patients who achieved HbA1c <7% and/or a composite endpoint of an HbA1c reduction ≥1% accompanied by a reduction in body weight (BW) ≥3% or ≥5% at the end of the study was recorded.
Important results at a glance
Of the 185 participants included, 90.8% completed the study and 77.3% remained on semaglutide therapy until the end of the study [3]. At baseline, the average age was 62 (SD 10.4) years, the HbA1c value was 7.7% (SD 1.5%) and the body weight was 95.6 kg (SD 17.6 kg). The mean BMI was documented as 33.2 kg/m2 (4.8 kg/m2). 56.2% of the participants received other glucose-lowering medication at the same time.
** SD = standard deviation
KI=confidence interval
The estimated mean changes from baseline to the end of the study were as follows [3]:
- HbA1cvalues: change of -0.9% (95% CI: -1.1; -0.7, p<0.0001)
- Absolute body weight: change of 4.7 kg (95% CI: -5.6; -3.8, p<0.0001)
- Relative body weight: reduction of -4.9% (95% CI: -5.7; -4.0, both p<0.0001)
- At the end of the study, 64.2% of the participants had an HbA1c value <7% and 37.8% and 28.3% achieved the composite endpoint of an HbA1c reduction ≥1% plus a KG reduction ≥3% and ≥5% respectively.
A total of 139 adverse events (AEs) were reported in 65 participants (35.1%) [3]. Most were mild or moderate, with gastrointestinal disorders being the most common (89 events in 50 participants). 31 AEs in 20 (10.8%) participants led to discontinuation of oral semaglutide. A total of six serious AEs were reported, which were probably not related to Semaglutide treatment.
Source: Novo Nordisk
Literature:
- Swissmedic: Arzneimittelinformation,
www.swissmedicinfo.ch, (last accessed 11/21/2023) - Husain M, et al.: PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. NEJM 2019; 381(9): 841–851.
- Kick A, et al.: Real-world use of oral semaglutide in adults with type 2 diabetes: Results from the PIONEER REAL Switzerland multicenter, prospective, observational study. Abstractband, Annual Meeting SGED/SSED, 16. –17.11.2023.
CARDIOVASC 2023; 22(4): 58
HAUSARZT PRAXIS 2023: 18(12): 37