For the first time, a targeted therapy for diabetic kidney disease is available. In the CREDENCE study, canagliflozin resulted in a clinically relevant and statistically significant risk reduction for renal failure and major cardiovascular events. SGLT-2 inhibitors inibriate renal sodium-dependent glucose transporters at the kidney and also play an important role in the tubuloglomerular feedback mechanism.
Globally, there is a trend toward an increase in the prevalence of type 2 diabetes (T2DM) and chronic kidney failure (CKD) [1,2]. Prevention and treatment of diabetic kidney disease in T2DM are essential, as it affects approximately 40% of patients and has been shown to increase cardiovascular risk and overall mortality risk [3–5]. The time to terminal renal failure is individually variable and can be delayed by adequate treatment. Renal disease is defined as a change in renal structure and function that has persisted for more than 3 months and is classified using the CGA scheme: cause (“cause”), GFR (G1-G5), and albuminuria (A1-A3). Since the early 2000s, little has happened until recently in terms of new specific treatment options for diabetic patients with nephropathy. It has been known for several decades that hypertension is a risk factor; in the 1990s, ACE inhibitors came on the market as antihypertensives, followed a few years later by angiotensin receptor blockers, which have become the cornerstone of treatment for diabetic nephropathy. It was not until 2019 that another breakthrough was achieved in the research of new therapy options explains PD Nilufar Mohebbi, MD, internist and nephrologist, Zurich practice and dialysis center [6].
Canagliflozin interferes with tubuloglomerular feedback mechanism
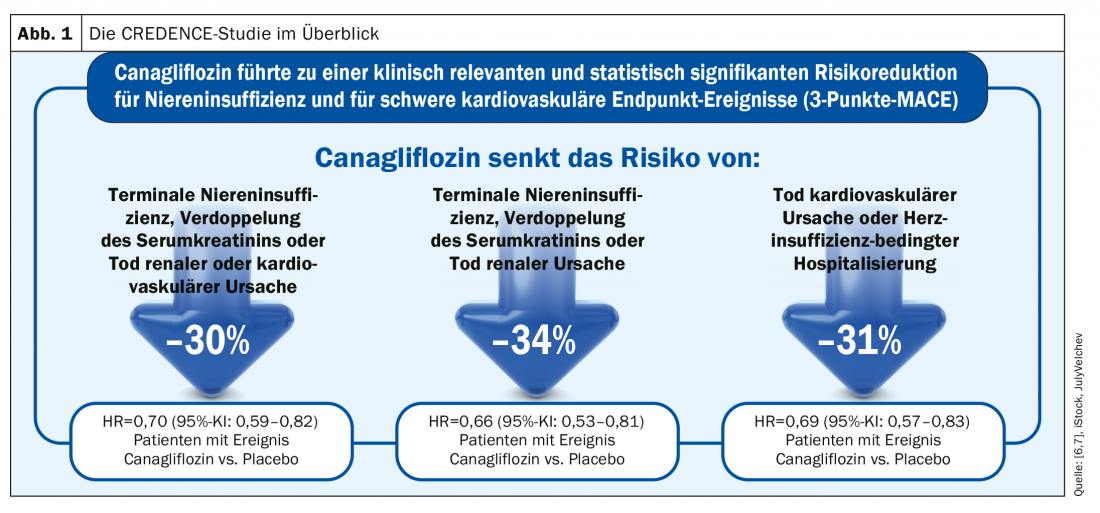
The CREDENCE study (“The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation study”) empirically demonstrated that the SGLT-2 inhibitor canagliflozin was able to slow the progression of renal failure [7]. The fact that this is interesting not only from a diabetological but also from a nephrological point of view has much to do with the mechanism of action of these substances. SGLT-2 inhibitors specifically inhibit the renal sodium-dependent glucose transporter SGLT-2 (“sodium dependent glucose co-transporter 2”) at the kidney, which is responsible for the reabsorption of glucose from the urine into the bloodstream, resulting in increased excretion of glucose in the urine. This is accompanied by osmotic diuresis (about 300 ml per day) and passager natriuresis [8]. Blood pressure decreases by an average of approximately 3 mmHg, and serum uric acid levels decrease by approximately 0.4 mg/dl. There is an increase in hematocrit, explainable by the stimulation of erythropoietin production in the kidney and initially partly by the reduction in plasma volume [9,10]. In the kidney, tubular stress decreases and hypertrophy of proximal tubules decreases, which can be explained by lower oxygen and energy demand due to less reabsorption of glucose [11]. SGLT-2 inhibitors also affect, among other things, tubuloglomerular feedback, the feedback mechanism of the renal tubular system that adjusts glomerular filtration rate (GFR) to tubular reabsorption. Activation of the tubuloglomerular feedback mechanism in the kidney with subsequent decrease in hyperfiltration and intraglomerular pressure leads to a 30% reduction in albuminuria and has an organ-protective effect [12]. As a result, the progression of kidney disease is delayed, manifested by a decreased drop in GFR over time [11]. This is reflected in a significantly lower proportion of patients requiring dialysis [13,14].
Evidence-based nephroprotective effect of canagliflozin.
Treatment with canagliflozin compared with placebo resulted in a 30% risk reduction for the composite primary endpoint (end-stage renal disease, doubling of serum creatine levels, death from a cardiovascular or renal cause) in the randomized, double-blind, multicenter phase III renal outcome study CREDENCE (n=4401) (Fig. 1 ). The risk for the renal-specific composite endpoint (end-stage renal disease, doubling of serum creatine levels, and death from a renal cause) was significantly reduced by 34% [7]. Safety data were comparable in both treatment groups. Based on the results of the CREDENCE study program, Invokana® (canagliflozin) received marketing authorization extension from Swissmedic in June of this year to reduce the risk of progression to diabetic kidney disease in adult patients with type 2 diabetes mellitus and albuminuria (ACR >300 mg/g). To date, it is the only SGLT-2 inhibitor with triple approval for blood glucose lowering, cardiovascular event prevention, and renal function protection in patients with T2DM [15].
KDIGO recommendations for therapy with SGLT-2 inhibitors
According to KDIGO (“Kidney Diseases Improving Global Outcomes”) 2019, the following therapeutic principles should be considered [16]: In type 2 diabetic patients with kidney disease and an eGFR ≥30 ml/min/1.73 m2, diabetes therapy should include an SGLT2 inhibitor. If there is a risk for the occurrence of hypovolemia, dose reduction of thiazide or loop diuretics should be considered before initiating therapy with an SGLT-2 inhibitor, and patients should be instructed to control their weight. A reversible decrease in glomerular filtration rate may occur upon initiation of therapy, but should not be a reason to interrupt therapy. Once therapy is started, it is recommended to continue – even if eGFR drops below 30 ml/min/1.73 m2 and as long as no uremic symptoms occur.
Source: FOMF (WebUp) 27.8.20
Literature:
- NCD Risk Factor Collaboration: Lancet 2016; 387: 1513-1530.
- Fu H, et al: Mol Metab 2019; 30: 250-263.
- Alicic RC, et al: Clin J Am Soc Nephrol 2017; 12(12): 2032-2045.
- Afkarian M, et al: J Am Soc Nephrol 2013 ; 24(2): 302-308.
- Liu M, et al: Eur Rev Med Pharmacol Sci 2014; 18(19): 2918-2926.
- Mohebbi N: New possibilities for the treatment of diabetic nephropathy – “the new kid on the block”, PD Dr. med. Nilufar Mohebbi, Internist and Nephrologist, Praxis und Dialysezentrum Zürich-City AG, FomF (WebUp), 27.8.2020.
- Perkovic V, et al: N Engl J Med 2019; 380 (24): 2295-2306.
- Cherney DZ, Kanbay M, Lovshin JA: Nephrol Dial Transplant 2020; 35: i1-2.
- Van Raalte DH, Bjornstad P: Nephrol Dial Transplant 2020; 35: i24-32.
- Gorriz JL, et al: Sodium-glucose cotransporter 2 inhibition toward an indication to treat diabetic kidney disease. Nephrol Dial Transplant 2020; 35: i13-23.
- Wanner C, Lopau K: Dtsch Arztebl 2020; 117(20): DOI: 10.3238/PersDia.2020.05.15.01,
- Vallon V, Thomson SC: Diabetologia 2017; 60: 215-225.
- Shivakumar O, Sattar N, Wheeler DC: Nephrol Dial Transplant 2020; 35: i43-47.
- Neuen Bl, Jardine MJ, Perdovic V: Nephrol Dial Transplant 2020; 35: i48-55.
- Swissmedicinfo: Invokana®, current expert information, www.swissmedicinfo.ch
- Kidney Deseases Improving Global Outcomes (KDIGO): KDIGO Clinical Practice Guideline on Diabetes Management in Chronic Kidney Disease. Public Review Draft December 2019. https://kdigo.org
HAUSARZT PRAXIS 2020; 15(9): 34-36 (published 9/19/20, ahead of print).