The more a patient with MS is limited in different areas of life by his increasing disability, the worse his health status becomes. In Switzerland, an oral MS treatment, teriflunomide, has just been approved and has been shown to significantly reduce the risk of disability progression in two phase III trials.
On November 5, 2013, it was announced that the MS therapeutic teriflunomide, available in oral form, received marketing authorization from Swissmedic under the name Aubagio® [1]. Aubagio® is indicated at a dosage of 14 mg/day for the treatment of adult patients with relapsing-remitting multiple sclerosis. At this year’s ECTRIMS congress, the experts had advocated an early, adequate choice of therapy tailored to the needs of the individual patient. The choice of therapy must also take into account the factor of treatment adherence, which is why the experts welcome the availability of further treatment options, including oral ones.
Significantly lower risk of disability progression
Teriflunomide inhibits proliferation of activated lymphocytes by inhibiting de novo biosynthesis of pyrimidine [2]. In addition, it seems to have various anti-inflammatory and immunomodulatory effects. In both the phase III TEMSO (“TEriflunomide Multiple Sclerosis Oral”) and TOWER (“Teriflunomide Oral in people With relapsing remitting multiplE scleRosis”) trials, teriflunomide significantly reduced the annual relapse rate and the risk of disability progression compared with placebo (Fig. 1) [3, 4].
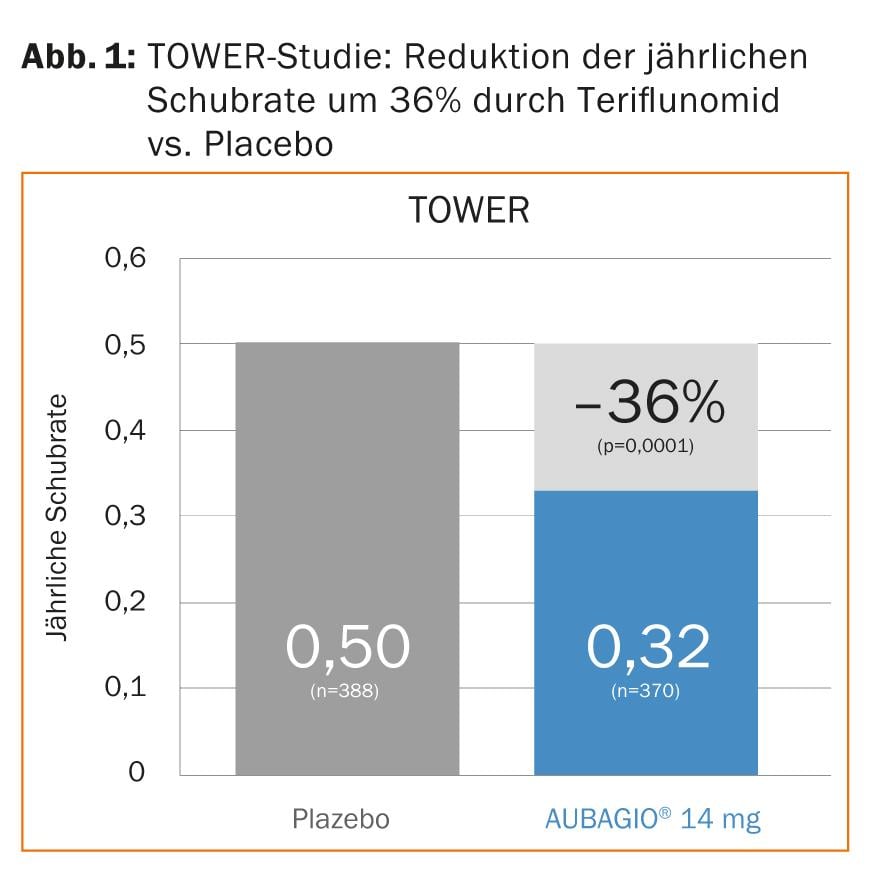
With TEMSO and TOWER, a reduction in the risk of disability progression was thus confirmed for the first time in two Phase III trials. A pooled analysis of data from TEMSO and TOWER presented at ECTRIMS also showed that comparable results could be achieved in patients with high disease activity (>2 relapses in the previous year) [5].
Most of the adverse events occurring with teriflunomide in the two studies were mild to moderate, resolved spontaneously, and rarely resulted in discontinuation of therapy. Data are now available from the extension phase of TEMSO for a treatment period of up to eight years [6]. They confirm the continued low relapse rate and the tolerability of the treatment.
Welcome new treatment option
Prof. Ludwig Kappos, MD, Basel, Switzerland, stated in the press release on the new approval of teriflunomide: “The fact that teriflunomide 14 mg has shown a positive effect on disability progression in two Phase III clinical trials underscores the importance of this compound as a new treatment option for patients with relapsing-remitting MS. As a new once-daily oral treatment option with a well-described side effect and safety profile, teriflunomide may be an attractive treatment option for patients dissatisfied with traditional injectable drugs.”
Literature:
- Press release Genzyme a Sanofi Company, November 5, 2013.
- Papadopoulou A, Kappos L, Sprenger T: Teriflunomide for oral therapy in multiple sclerosis. Expert Rev Clin Pharmacol 2012; 5: 617-28.
- O’Connor PW, et al: Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 2011; 365: 1293-1303.
- Kappos L, et al: The efficacy and safety of teriflunomide in patients with relapsing MS: results from TOWER, a phase III, placebo-controlled study. Oral presentation: 28th Congress of the European Committee of Treatment and Research in Multiple Sclerosis 2012.
- Kappos L, et al: Pooled efficacy data from two phase 3 placebo-controlled trials of oral, once-daily teriflunomide. Ectrims 2013, Abstract P618.
- Freedman M, et al: Long-term safety and efficacy of teriflunomide in patients with relapsing forms of multiple sclerosis in the TEMSO extension trial. Ectrims 2013, Abstract P544.
- Miller A, et al: TOPIC main outcomes: efficacy and safety of once-daily oral teriflunomide in patients with clinically isolated syndrome. Ectrims 2013, Abstract 99.
InFo Neurology & Psychiatry 2013; 11(6): 48.