Digitization in healthcare holds many opportunities, especially for disease management of chronic diseases. When digital applications are integrated into a personalized and long-term treatment plan, it can help improve care for rheumatoid arthritis sufferers. This is one of the core statements of current studies and expert recommendations. But what does this mean in concrete terms?
The use of digital health applications in routine rheumatology care has increased in recent years, and the coronary pandemic has exacerbated this trend [1]. Telemedicine and digital devices, such as wearable activity trackers and artificial intelligence are forward-looking topics (box) [2]. Studies show that digital applications can improve the self-management of sufferers in particular. This applies, for example, to patients with inflammatory arthritis, as shown by the recommendations of the European Alliance of Associations for Rheumatology (EULAR) published last year [3]. Prof. Andrea Marques, Higher School of Nursing of Coimbra, Centro Hospitalar e Universitário de Coimbra EPE (Portugal), gave an update on the topic of modern technologies for the management of rheumatologic diseases in her presentation at the EULAR Annual Congress 2022 [4].
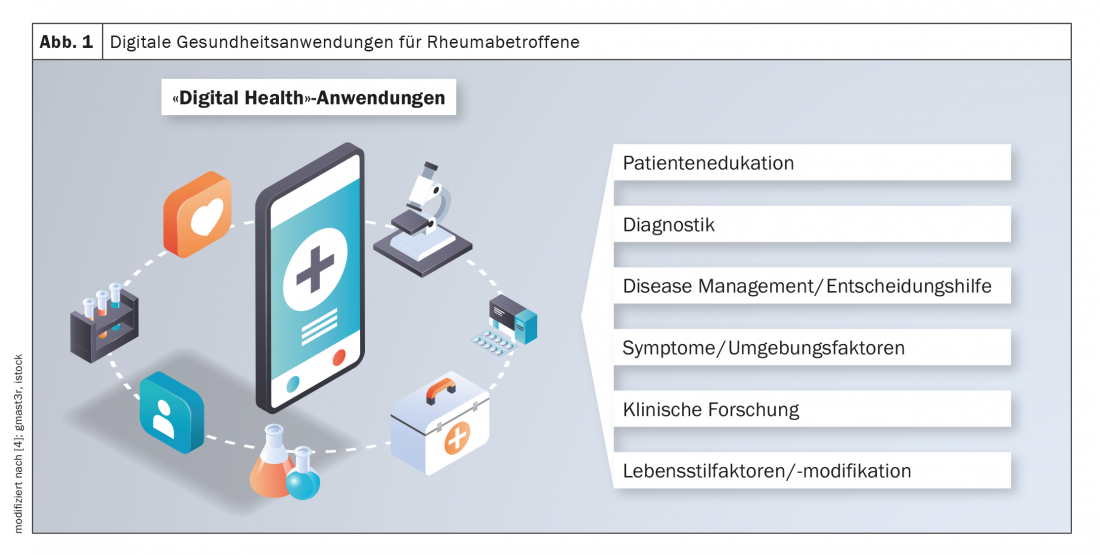
The following digital health apps are currently available for rheumatoid arthritis:
- Virtual consultation: telephone, video-assisted, with or without “wearables” and apps
- Apps and web-based interventions for patients: Patient Reported Outcome Measures (PROMS), medication diary, nutrition and lifestyle tracker, disease information.
- Wearable sensors: Activity and sleep
- tracker
- “Digital therapeutics”: tools that address lifestyle factors (e.g., adherence, medication adherence)
- Artificial intelligence and machine learning: predictive models for risk stratification, automated data collection and summarization.
- Electronic patient dossier: decision support (e.g. disease activity measurement), visualization tools
|
Better drug adherence thanks to robotic technology Studies show that in developed countries, more than half of patients with chronic diseases do not follow doctor-recommended medication regimens [11]. In addition to web applications that address this problem (e.g., reminders via SMS or e-mail), there are innovative smart technologies that are designed to make everyday life easier for patients with a chronic illness. Pillo is a voice-controlled health assistant that uses adaptive artificial intelligence to help patients maintain safety, independence and well-being at home [12]. This includes the dispensing of medication at a set time, which can be programmed by the user themselves or their caregiver or healthcare provider. To ensure that only those authorized have access to the medication, facial recognition or a PIN number is used [11]. For healthcare organizations, the Pillo deviceand platform provide continuous interaction with patients, giving providers up-to-the-minute insight into their daily routines – including verifiable medication adherence data [12]. |
“Wearable devices”: high patient benefit
Implementing digital health apps can help improve patient-reported outcome measures (PROMs) as well as patient-reported experience measures (PREMs). “The patient feels more involved in decision making,” explains Prof. Marques, adding, “Wearable devices are most commonly used by our patients.” Activity trackers provide powerful predictive analytics about impending deteriorations in health or functional status by capturing changes in activities of daily living (ADLs). CarePredict is an activity tracker that is worn on the dominant hand and detects activities such as eating, showering and going to the toilet based on characteristic movement patterns [5,6]. Walks and sleep patterns are also recorded. This tracking can predict health risks such as exposure to falls or improper nutrition. The Device includes a touch-button call system for real-time communication with caregivers [6].
App-supported patient-oriented treatment
Cliexa-RA is a graphical app for recording pain and following up on treatments [4,7]. This valuable tool can be optimally integrated into disease management. The platform offers providers the ability to collect data not only on pain progression, but also on the impact on daily functionality, quality of life, and social factors. Consistent functional assessment of the patient promotes a more comprehensive understanding of the impact of their disease on their overall well-being and supports the patient-centered care model. In addition, Cliexa-RA offers a range of visual and functional scales for gold standard assessments, including DAS-28, CDAI, SDAI and RAPID3. Providers save time in assessment recording, documentation, and data collection over the long-term, leaving more time for individualized patient care [7].
“Digital therapeutics” and “DiGa” as medical devices
Digital health applications that have been officially approved by the US Food and Drug Administration (FDA) are called “digital therapeutics” [4]. Evidence-based software applications have a proven clinical benefit in helping patients self-manage, thereby improving their quality of life and other clinical outcomes [8]. Digital tools such as mobile devices, apps, sensors, virtual reality, the Internet and other tools are used for this purpose. One example of “digital therapeutics” is the reSET app [9]. There are also developments in this regard in Europe, where the marketing of a medical product is subject to strict regulations. In Germany, those insured by the statutory health insurers have already been able to benefit from the “app on prescription” since 2020. These are physician-prescribed digital health applications (“DiGa”) that meet the legal guidelines of a medical device. In the meantime, the hurdles for approval have risen even further. On May 26, 2021, the Medical Device Regulation (MDR) came into force in Germany, replacing the previous Medical Device Derivation (MDD) Directive [13].
And what is the situation in Switzerland? eHealth Suisse, the competence and coordination office of the federal government and the cantons, has published the “Guide for app developers, manufacturers and distributors” in 2022. This provides practical guidance on the conditions under which an app is considered a medical device and what the regulatory requirements are in this regard [10].
Congress: EULAR Annual Meeting 2022
Literature:
- de Thurah A, et al: Future challenges in rheumatology – is telemedicine the solution? Ther Adv Musculoskelet Dis 2022 Mar 17; 14:1759720X221081638.
- Knitza J, et al.: Position Paper of the Digital Rheumatology Commission of the German Rheumatology Society: Tasks, goals and perspectives for modern rheumatology. Z Rheumatol 2020; 79: 562-569.
- Nikiphorou E, et al: 2021 EULAR recommendations for the implementation of self-management strategies in patients with inflammatory arthritis. Ann Rheum Dis 2021; 80(10): 1278-1285.
- “Digital-Health and Tailored Interventions for Improving Patients’ Adherence: Opportunities and Challenges,” Health Professionals in Rheumatology, Prof. Andrea Marques, EULAR Annual Meeting, June 04, 2022.
- Kataria S, Ravindran V: Digital health: a new dimension in rheumatology patient care. Rheumatology International 2018; 38:1949-1957.
- CarePredict, www.carepredict.com, (last accessed Jun. 23, 2022).
- cliexa-RA, www.cliexa.com/cliexamobile/rheumatoid-arthritis, (last accessed Jun. 23, 2022).
- European Data Protection Supervisor, https://edps.europa.eu/press-publications/publications/techsonar/digital-therapeutics-dtx_de, (last accessed June 23, 2022).
- reSET, https://peartherapeutics.com/products/reset-reset-o, (last accessed Jun. 23, 2022).
- eHealth Suisse: Guide for app developers, manufacturers and distributors, www.e-health-suisse.ch/fileadmin/user_upload/Dokumente/D/Leitfaden_e-Health_Suisse_fuer_App_Entwickler.pdf, (last accessed 23.06.2022).
- Faisal S, Ivo J, Patel T: A review of features and characteristics of smart medication adherence products. Can Pharm J (Ott). 2021 Jul 30;154(5):312-323. https://journals.sagepub.com/doi/pdf/10.1177/1715163521103419
- Pillo Health, https://pillohealth.com, (last accessed Jun. 23, 2022).
- Medical Software and Medical Apps: Qualification, Classification, and Approval as Medical Devices, 07.12.21, https://meso.vde.com/de/software-medizinprodukt, (last accessed 23.06.2022).
HAUSARZT PRAXIS 2022; 17(7): 36-37