On August 21, 2014, a stroke symposium was held at the Inselspital in Bern, organized by the University Department of Neurology. The first presentations focused on the structure and certification of stroke units. After the break, experts provided information on various aspects of stroke therapy. We report on six of the total of nine presentations.
(ee) Helsinki University Central Hospital’s neurological emergency unit serves a catchment area of 1.6 million inhabitants (all of Finland: about 5.5 million people). Prof. Turgut Tatlisumak, M.D., Chief Physician at the Stroke Center in Helsinki, showed how he was able to improve the average time from symptom onset to thrombolysis in his hospital for stroke patients. The goal of the measures was to achieve recanalization in as many patients as possible.
Strong rescue chains are important
Thrombolysis must occur in the first 4.5 hours after symptom onset – the sooner, the better. This time is divided into the “onset-to-door” time (symptom recognition, emergency call, arrival at the ambulance, evaluation of the patient, travel to the emergency department) and the “door-to-needle” time (admission of the patient, history and examination, laboratory, imaging, assessment of thrombolysis indication, obtaining consent, initiation of treatment). All links in this chain must be optimized.
Prof. Tatlisumak emphasized that it is very important to keep the “door-to-needle” time short, even if a patient arrives at the hospital quickly and theoretically has more time for in-hospital evaluations. At Helsinki University Central Hospital, about 30% of stroke patients receive thrombolysis, and the “door-to-needle” time has decreased from three hours in 1998 to a current average of 20 minutes. A neurologist is present at the hospital at all times to receive the stroke patient and subsequently make all decisions up to thrombolysis. These physicians attend special trainings.
Measures must also be taken for the time outside the hospital: constant information of the population about the symptoms and the correct procedure in case of suspected stroke, further training of paramedics, organization of the rescue chain, etc. “Stroke patients should only be admitted to hospitals that have access to a stroke unit 24 hours a day,” the speaker demanded. Valuable work is already being done during the patient’s transport to the hospital (setting up access, laboratory tests, monitoring vital signs, finding out telephone numbers of relatives, notifying the hospital, etc.).
In Germany, there are currently trials in two cities with mobile stroke units, i.e. special outpatient clinics equipped with CT, laboratory and trained staff. The goal is to bring the stroke unit – and the opportunity for thrombolysis – to the patient, rather than the patient to the stroke unit. Whether this will halve the time to thrombolysis and improve patient outcome is currently unclear.
Endovascular therapy options for stroke.
Prof. Jan Gralla, MD, Neuroradiology, Inselspital Bern, discussed the various endovascular therapies. In all of them, the goal is to restore brain perfusion, preserve penumbra brain functions, and thus achieve a smaller neurological deficit for the patient. Depending on the procedure, different time windows are open: 4.5 hours for i.v. lysis, six hours for intra-arterial lysis, and eight hours for mechanical thrombectomy. One problem with i.v. lysis is that large thrombi (>8 mm) in large vessels are not dissolved. These thrombi are amenable to mechanical thrombectomy – which is also not easy to accomplish, however, because the cerebral vessels are narrow and very tortuous.
Thrombectomy procedures are divided into distal thrombectomy (basket is deployed behind the thrombus) and the more modern procedure with stent retriever (stent is placed parallel to the thrombus and then deployed, subsequently removed with the thrombus). In the stent retriever procedure, recanalization rates are high (up to 80%) with good patient outcome. The procedure is also fast: on average, 25-45 minutes elapse from groin stitch to recanalization. There are now many different stent retrievers on the market. However, the efficiency has not yet been proven by means of randomized-controlled trials.
The question remains open as to which procedure to use to treat patients who can be treated within 4.5 hours of symptom onset. The three available options were presented in three studies in the NEJM in February 2013, and there was no difference in efficacy. However, these studies had several weaknesses, the speaker pointed out. Currently, the SWIFT-PRIME trial is ongoing, treating patients with i.v. lysis or with i.v. lysis followed by mechanical recanalization. However, the initial conditions are also decisive for the outcome. Prof. Gralla put it as follows: “You can only save what is still there” and thus once again emphasized the relevance of an optimized rescue chain.
Atrial fibrillation, anticoagulation and stroke
Prof. Dr. med. Heinrich Mattle, Inselspital Bern, provided information on the correlation between atrial fibrillation (VHF) and stroke. Most patients with VCF are initially seen by an internist, for example, because of hypertension or heart failure. “Only” about 10% have a stroke or transient ischemic attack (TIA) as their first symptom. Patients with stroke and VHF are on average older than stroke patients without VHF and have territorial infarctions more often. Anticoagulation reduces the risk of stroke by two-thirds. Whether there is an indication for anticoagulation is evaluated using the CHA2DS2-VASc score. New anticoagulants (dabigatran, rivaroxaban, apixaban) work better than warfarin for prevention of hemorrhagic strokes. Dabigatran reduces the risk of stroke better than Marcoumar® and causes fewer hemorrhagic side effects.
Prof. Mattle answered some practical questions about anticoagulation in cerebral stroke:
- When to start anticoagulation after TIA or stroke? Patients receive aspirin (not heparin) for the first two weeks. Thereafter, one proceeds according to the “1-3-6-12” rule: For TIA, anticoagulation starts immediately (day 1), for minor stroke after three days, for moderate infarction after six days, and for severe infarction after 12-21 days.
- Should we combine anticoagulation and antiplatelet agents? Triple therapy is not recommended because of a relatively high risk of bleeding. However, anticoagulation plus antiplatelet agents (clopidogrel does best in trials) is reasonable, even for coronary patients with stenting.
- Should anticoagulation be resumed after cerebral hemorrhage? In principle, one should know the cause of the bleeding in order to make a decision here. For bleeding deep in the brain, anticoagulation can be restarted after 10-14 days. To prevent rebleeding, keep INR <3, lower blood pressure, and do not combine antiplatelet drugs. For superficial bleeding, the risk of bleeding with anticoagulation may be too great, but evidence is lacking.
- Should we anticoagulate patients with cavernomas? If the patient has not bled, you may anticoagulate because the risk of bleeding is small. However, if the cavernoma has bled, there is a significant risk of new bleeding; in these patients, the cavernoma should be surgically removed before anticoagulation.
- Should neurosurgical patients be anticoagulated? Ten weeks after subdural hematoma or craniotomy, these patients receive one of the new anticoagulants. In the period prior to this, patients are treated with heparin.
- Can patients at high risk for falls be anticoagulated? The risk of falls is often overestimated, and usually the benefit of anticoagulation is greater than the risk of bleeding after a fall. A Swiss study found that major, life-threatening bleeding was no more common in fall patients than in others. A recent study shows that in patients with a score of 0-3 on the CHADS score, the risk of anticoagulation is greater than the benefit; however, anticoagulation should be used above a score of four on the CHADS score.
- What to do in patients with contraindication to anticoagulation? They should be clarified by a cardiologist, because atrial ear closure may be possible and useful.
Does cryptogenic stroke exist?
If standard investigations to find the cause of stroke (ECG, 24-hour ECG, echo, imaging, laboratory, etc.) are negative, cryptogenic stroke is postulated (in about 20-40% of stroke patients). Simon Jung, MD, Bern, recommended that the term “embolic stroke of undetermined cause” should be used, because with extended investigations a cause can be detected in the majority of these patients (Fig. 1).
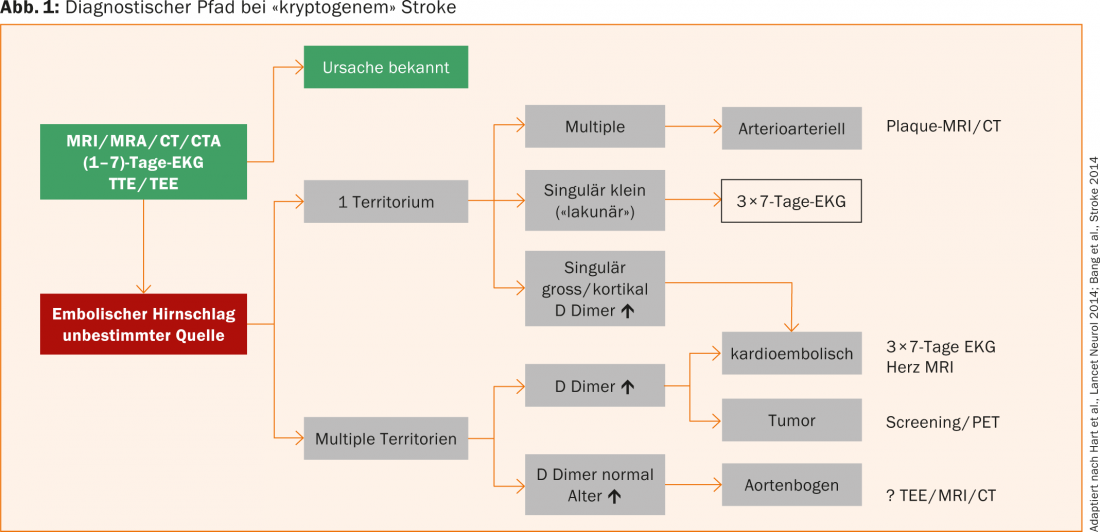
First, we distinguish whether the ischemic lesion(s) affect one or more territories. Multiple ischemias in a territory are suspicious for an arterioarterial event, even in the presence of non-significant hemodynamically stenosing plaques. In these patients, a search for the unstable plaque with MRI/CT is reasonable. In singularly large or cortically located infarcts or when distributed over multiple territories and when D-dimers are markedly elevated, a cardioembolic genesis is likely and should be sought with vigor. Recent studies show that detection of intermittent VHF increases with recording time. Therefore, it is recommended to perform a 7-day ECG and repeat it twice if it is negative.
If multiple territories of ischemic lesions are affected and D-dimers are elevated, a tumor-associated coagulopathy in particular should be considered in addition to a cardioembolic etiology. With lesions in multiple territories and normal D-dimers, plaques may be present in the aortic arch, usually in patients who tend to be elderly. The workup is performed by transesophageal echocardiography, MRI, or CT.
Extended diagnostics can significantly reduce the proportion of patients with an unknown cause of stroke, which also results in a relevant change in therapy (e.g., anticoagulation or tumor therapy) for a large proportion of these patients.
Blood pressure management in acute stroke
“After an ischemic stroke, blood pressure is elevated in most patients,” said PD Urs Fischer, MD, Inselspital Bern. “Eighty-one percent of patients have a systolic pressure >140 mmHg. The mechanism behind the blood pressure elevation is unclear.” Suspected causes include disruption of cerebral autoregulation, neuroendocrine factors, dysregulation due to brain tissue death, psychological stress, etc. In some cases, the elevated blood pressure is also the cause of the stroke. Post-stroke blood pressure has a prognostic component: the lower or higher the pressure, the worse the patient’s fate (U-shaped curve).
Whether and how much to lower blood pressure is controversial. According to the SCAST study, there is no difference in outcome whether blood pressure is lowered or not. However, in this study, hemorrhages and ischemias were assessed together, which is problematic. In the 2013 CATIS study, only patients with ischemic strokes were studied. Again, there were no overall differences in outcome. However, the time to follow-up was only 14 days, and thus no statement can be made about the long-term effects of blood pressure reduction in the acute phase. However, in the subacute phase, blood pressure can probably be lowered without harming the patient.
Whether antihypertensives need to be paused in acute cerebral stroke was a question the COSSACS trial sought to answer. Here, too, the fate of the patients did not depend on the stop or the from continuing hypertension treatment, however, the study had too little power to make a definitive statement. At least the results suggest that blood pressure medications can be continued in acute stroke, which makes sense because patients often also have heart disease. In principle, the AHA guidelines apply to blood pressure lowering after ischemic stroke: blood pressure lowering by a maximum of 15% and only for systolic values >220 mmHg or diastolic values >120 mmHg.
With cerebral hemorrhage, there is often concern that the hematoma may increase with high blood pressure. In the INTERACT study, blood pressure in cerebral hemorrhage was lowered below 140 mmHg. This measure was safe, but there was not much difference in fate compared with the group of patients without blood pressure lowering. The speaker interpreted the results cautiously: “Blood pressure reduction is probably not harmful and may lead to an improvement in clinical fate – but this is only true for cerebral hemorrhages with small hematoma volumes.”
To date, many randomized trials of blood pressure and stroke have had major methodological weaknesses. In future studies investigating the potential of blood pressure reduction in cerebral stroke, other factors such as penumbra, premorbid blood pressure, site of vessel occlusion, etc., need to be considered, and only patients with a consistent clinical picture (ischemia or hemorrhage) should be included.
Management of intracerebral hemorrhage
“In the management of intracerebral hemorrhage, unfortunately, there is hardly any evidence for anything!” This is how Prof. Jürgen Beck, MD, Inselspital Bern, introduced his lecture. Intracerebral hemorrhage is more common in the elderly. It is recommended that patients’ blood pressure be lowered to 160 mmHg (180 mmHg for intracranial pressure). However, the INTERACT trial showed that there was no difference in outcome or incidence of rebleeding between patients with aggressive blood pressure lowering (<140 mmHg) and patients without blood pressure lowering.
There is also no evidence for surgical removal of the hemorrhage. In the STICH study, there was no positive effect from hematoma removal for the entirety of patients. In the subgroup of deeply comatose patients, surgery even increased the risk of poor outcome! There is a trend for surgery to improve outcome only in superficial and lobar hemorrhage and in patients scoring 9-13 on the GCS (i.e., patients who are not fully awake but also not deeply comatose). The extent of the hemorrhage also plays a role: in the case of deep hemorrhages with a volume >30 ml, patients have little chance of survival.
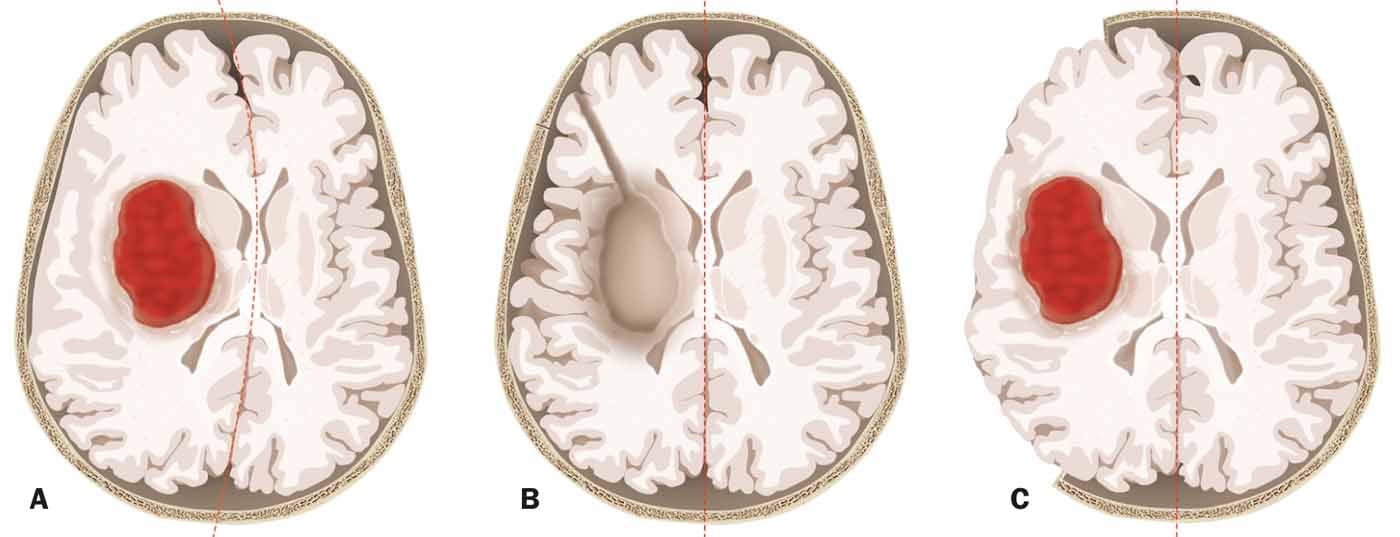
The speaker emphasized that when a bleed is removed, the procedure itself also often puts the patient at risk. Unlike tumor surgery, for example, hemorrhage removals in an emergency situation are likely to damage functional brain parenchyma. An alternative is decompressive craniectomy, in which the parenchyma is spared (Fig. 2) . There is evidence that craniectomies for cerebral hemorrhage can reduce mortality. In this regard, a new Swiss study is planned (SWITCH) to investigate whether craniectomy can not only reduce mortality but also improve outcome. Recruitment of the first patients can hopefully begin this year.

Source: Stroke Symposium, August 21, 2014, Bern