First-line chemotherapy followed by avelumab (BAVENCIO®)-maintenance therapy is the standard of care for patients with locally advanced or metastatic urothelial carcinoma who have not progressed on platinum-based chemotherapy, according to the guidelines [1–4]. Which patient groups benefit most from maintenance therapy with avelumab? And how long should maintenance therapy last? These questions were discussed at the satellite symposium “First-line BAVENCIO® maintenance therapy: routine in advanced/metastatic urothelial carcinoma” during the annual meeting of the DGHO, OeGHO, SGMO and SGH in Vienna on October 7, 2022.
Urothelial carcinoma is the most common form of bladder cancer and often progresses within a few months despite a good response to platinum-based chemotherapy (ChT) [5]. The pivotal, randomized phase 3 JAVELIN Bladder 100 trial evaluated first-line maintenance therapy with avelumab + BSC (best supportive care) versus BSC alone in patients with locally advanced or metastatic urothelial carcinoma who were not progressive on first-line ChT [5]. Patients who received prior ChT of 4 to 6 cycles and who had achieved at least stable disease (SD) status were eligible for the study [5]. The primary endpoint of the JAVELIN Bladder 100 study was median overall survival (OS): At long-term follow-up of ≥2 years, this showed a clear OS benefit of 8.8 months in the avelumab + BSC arm versus BSC alone (HR 0.76; 95% CI: 0.631-0.915) [6]. Avelumab + BSC also showed a benefit in the secondary endpoint, median progression-free survival (PFS), with 5.5 months (95% CI: 4.2-7.2) versus 2.1 months (95% CI: 1.9-3.0) with BSC alone (HR 0.54; 95% CI: 0.457-0.645, p<0.0001) [6]. At this year’s DGHO congress, Prof. Dr. Lukas Weiss from the University Hospital of Internal Medicine Salzburg, PD Dr. Richard Cathomas from the Cantonal Hospital Graubünden, and PD Dr. Maria De Santis from Charité Universitätsmedizin Berlin discussed subgroup analyses from the JAVELIN Bladder 100 study, current real-world data on avelumab, and case studies from their daily clinical practice.
“Even patients with an apparently complete remission benefit from maintenance therapy.” Prof. Lukas Weiss, MD
What role does the patient’s general condition play?
In his presentation, Prof. Dr. Lukas Weiss introduced the current ambispektive real-world study AVENANCE [7]. In general, patients here were more likely to be included with worse general health and prognosis than in the JAVELIN Bladder 100 trial: 12.6% had an ECOG PS 2 and 82.7% of patients in AVENANCE showed visceral metastases [7]. In the JAVELIN Bladder 100 trial, this was the case in 0.3% and 54.6% of patients, respectively [5]. The median OS of patients from the interim analysis of AVENANCE was 20.7 months (15.2-NE) after a median follow-up of 13.5 months [7]. In the JAVELIN Bladder 100 trial, patients achieved a median OS of 23.8 months (19.9-28.8) in the avelumab + BSC arm versus 15.0 months (13.5-18.2) in the BSC arm at ≥2 years of follow-up [6]. Prof. Weiss concluded that the benefit of avelumab maintenance therapy is reinforced with the real-world data and that efficacy should be observed regardless of the patient’s general condition. Referring to the JAVELIN Bladder 100 trial, PD Dr. Cathomas also emphasized, “The Forrest plot for OS is on the right side for all subgroups” [6].
“All subgroups studied appear to benefit from avelumabmaintenance therapy benefit.” PD Richard Cathomas, MD
What is the significance of the previous ChT and the response to it?
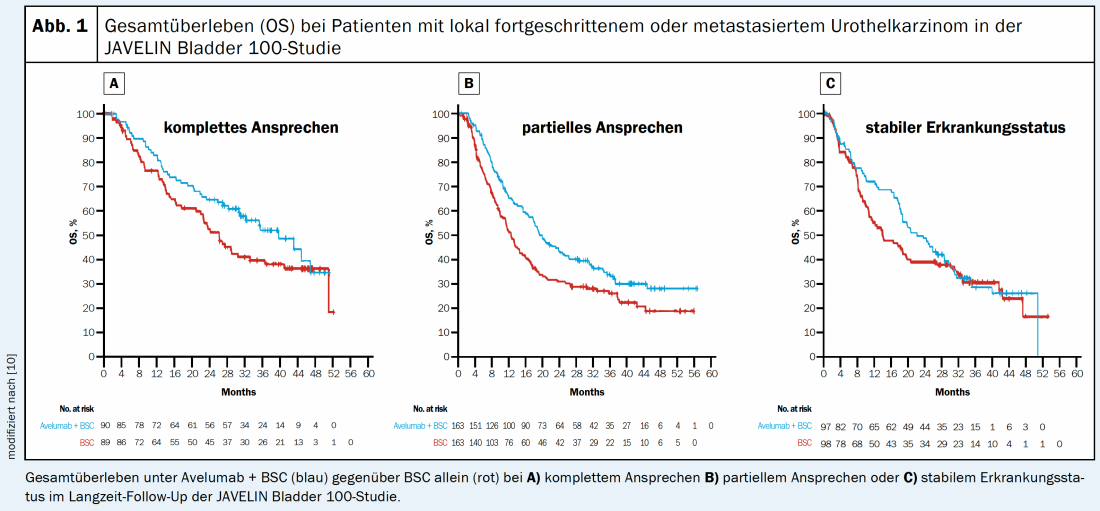
Subgroup analyses from the JAVELIN Bladder 100 trial indicate that avelumab maintenance therapy after cisplatin-based ChT performs similarly to carboplatin-based ChT: median OS with avelumab + BSC was 25.3 months (HR 0.69; 95% CI: 0.51-0.94) and 19.9 months (HR 0.66; 95% CI: 0.47-0.91) in the subgroups, respectively [8]. A post-hoc analysis of the JAVELIN Bladder 100 trial also showed that the number of previous ChT cycles (between 4 and 6) did not affect the efficacy of maintenance therapy [9]. The OS benefit of avelumab maintenance therapy was seen in patients with complete remission (CR) as well as in patients with partial remission (PR) or SD after prior ChT (Fig. 1) [10].
Does avelumab maintenance therapy work regardless of patients’ PD-L1 status?
In their case studies, speakers PD Dr. De Santis and PD Dr. Cathomas demonstrated that PD-L1 status had no impact on the efficacy of avelumab maintenance therapy in the respective patient cases. This is consistent with the results of the JAVELIN Bladder 100 study. The OS benefit of maintenance therapy was observed in the long-term follow-up of the JAVELIN Bladder 100 trial in both the PD-L1-positive and PD-L1-negative patient populations [6]. Treatment with avelumab is approved and recommended in guidelines for patients with locally advanced or metastatic urothelial carcinoma who did not progress on first-line CT, regardless of PD-L1 status [1–4]. PD Dr. Cathomas additionally addressed the duration of treatment with Avelumab in his case report. According to the recommendations, avelumab maintenance therapy is provided until or unless unacceptable toxicity symptoms occur [1]. PD Dr. De Santis demonstrated that a new second-line therapeutic option is indicated in the setting of relapse on avelumab maintenance therapy [4].
Conclusion
Real-world data from the AVENANCE trial shown at the DGHO Congress reaffirm the OS benefit with avelumab maintenance therapy in a broad and fragile patient population with locally advanced or metastatic urothelial carcinoma without progression on prior ChT [7]. Patient cases from the three experts, Prof. Dr. Weiss, PD Dr. De Santis, and PD Dr. Cathomas, as well as subgroup analyses from the JAVELIN Bladder 100 trial suggest that the efficacy of avelumab maintenance therapy is independent of carboplatin/cisplatin, of 4-6 ChT cycles or of the measure of response in previous ChT, and independent of the patient’s PDL1 status [6,8–10].
“New second-line therapy options are available for patients with relapse on maintenance therapy.” PD Maria De Santis, MD

Imprint
Reporting: Dr. sc. nat. Katja Becker
Source: DGHO Congress 2022; October 7, 2022; Vienna, Austria.
This article was written with the financial support of Merck (Schweiz) AG, Zug and Pfizer AG, Zurich.
CH-AVEBL-00173 November 2022
© Prime Public Media AG, Zurich 2022
Brief technical information of BAVENCIO®:
BAVENCIO®(20 mg/ml avelumab, fully human immunoglobulin G1 monoclonal antibody). I: For the treatment of patients with metastatic Merkel cell carcinoma (MCC). As monotherapy for the first-line maintenance treatment of patients with locally advanced or metastatic urothelial carcinoma (UC) whose disease has not progressed with first-line platinum based induction chemotherapy. PO: 10 mg/kg body weight once every 2 weeks, administered intravenously over 60 minutes until disease progression or unacceptable toxicity. Premedi – cation with an antihistamine and with paracetamol at least prior to the first 4 infusions. Handling instructions and guidelines for withholding or discontinuation of the therapy are to be strictly adhered to. CI: Hypersensitivity to avelumab or to any of the excipients. W: Immune-related adverse reactions including haemophagocytic lympho histiocytosis, immune-related pneumonitis, immune-related hepatitis, immune-related colitis, immunerelated pancreatitis, immune-related myocarditis, immune-related endo crinopathies (hypothy-roidism or hyperthyroidism, adrenal insufficiency, type 1 diabetes mellitus), immune-related nephritis. Infusion-related reactions which might be severe. Adverse events in transplant recipients, embryofoetal toxicity. IA: None known. Most common UE: Immune-related adverse reactions and infusion-related reactions. Headache, dizziness, neuropathy peripheral, hypertension, hypotension, dry mouth, increased liver values, fatigue, pyrexia, asthenia, chills, influenza like illness, nausea, vomiting, diarrhoea, constipation, decreased appetite, weight decreased, hyponatraemia, abdominal pain, urinary tract infection, dyspnea, cough, pneumonitis, dysphonia, rash, pruritus, dry skin, anemia, lymphopenia, thrombocytopenia, hypothyroidism, hyperthyroidism, back pain, arthralgia, myalgia, creatinine, amylase or lipase increased, peripheral oedema. P: 1 + 4 vials of 10 ml (200 mg avelumab). [A] For further information, see www.swissmedicinfo.ch. AUG21
Literature:
- Specialist information BAVENCIO® (avelumab). www.swissmedicinfo.ch, current status.
- Cathomas R, et al: The 2021 Updated European Association of Urology Guidelines on Metastatic Urothelial Carcinoma. Eur Urol 2022; 81(1): 95-103.
- National Comprehensive Cancer Network® (NCCN Guidelines®). Bladder Cancer (version 2.2022). www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Last accessed: October 2022.
- Powles T, et al: Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2022; 33(3): 244-258.
- Powles T et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. NEJM 2020; 383(13): 1218-1230.
- Powles T, et al: Avelumab first-line (1L) maintenance for advanced urothelial carcinoma (UC): long-term follow up results from the JAVELIN Bladder 100 trial. Presented at ASCO GU February 2022.
- Barthélémy P, et al: Preliminary results from AVENANCE, an ongoing, noninterventional realworld, ambispective study of avelumab first-line maintenance treatment in patients with locally advanced or metastatic urothelial carcinoma. Presented at the ESMO congress, September 9-13, 2022; Paris, France; Hybrid Meeting. Poster 1757P.
- Grivas P, et al: Avelumab first-line maintenance in locally advanced or metastatic urothelial carcinoma: Applying clinical trial findings to clinical practice. Cancer Treat Rev 2021; 97: 102187.
- Loriot Y, et al: Avelumab first-line maintenance plus best supportive care (BSC) vs BSC alone for advanced urothelial carcinoma: JAVELIN Bladder 100 subgroup analysis based on duration and cycles of first-line chemotherapy. Presented at the ASCO Genitourinary Cancers Symposium; 11-13 February 2021; Virtual Meeting. Abstract 438.
- Valderrama BP, et al: Avelumab first-line maintenance for advanced urothelial carcinoma: long-term outcomes from JAVELIN Bladder 100 in subgroups defined by response to first-line chemotherapy. Presented at the ASCO Annual Meeting, June 3-7, 2022; Chicago, IL. USA.Hybrid Meeting. Poster 4559.
The references are available on request.