The FOPH and the Federal Commission on Vaccination recommend vaccination against herpes zoster with the adjuvanted subunit vaccine for healthy people aged 65 and over and for patients with immunodeficiency aged 50 and over or, where appropriate, from the age of 18. The basic vaccination against chickenpox (varicella) is included in the vaccination schedule for infants aged 9 and 12 months. People aged between 13 months and 39 years who have not been vaccinated and who have not yet contracted varicella are advised to have a booster vaccination.
Over 95% of people in Switzerland are infected with chickenpox (varicella) in childhood, but usually only contract a mild fever and itchy skin rash or a generalized exanthema [1,2]. Of those who first fall ill at the age of ≥16 years, around 50 have to be hospitalized each year due to complications and around 20 per 100,000 die as a result, which is around 10-20 times more often than in younger children [3]. The varicella zoster viruses remain in the body for life and can be reactivated decades later and cause herpes zoster (shingles) [2].
Reactivation of varicella viruses: around one in three suffer complications
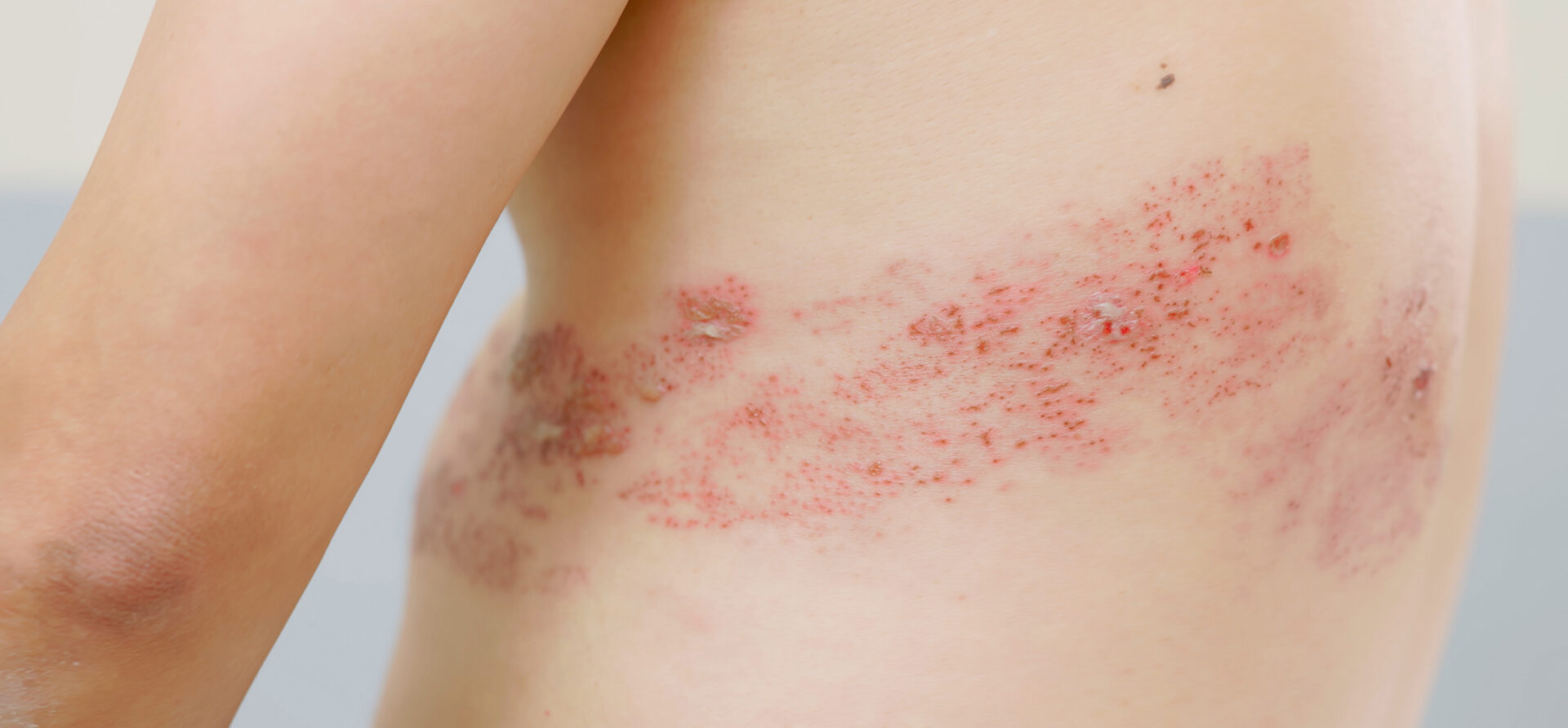
Around a third of those who have had chickenpox will develop shingles later in life [3]. The prevalence increases from the age of 50 and is higher in immunocompromised people [4,5]. In Switzerland, over 20,000 consultations per year are associated with shingles, half of which affect people over 65 [6]. Herpes zoster manifests itself as a painful and/or burning rash that dries up after a few days and forms a scab that eventually falls off [6]. Complications occur in around 30% of cases; eye infections in particular can be serious and are associated with a risk of blindness (Box) [2,6]. In addition, chronic pain can occur weeks or months after shingles; in 20% of patients over 65 years of age, this condition lasts for more than three months (post-zoster neuralgia) [2,6].
| Complications of herpes zoster: Elderly and immunocompromised people are particularly at risk The most common complication of herpes zoster (HZ) is post-herpetic neuralgia (PHN). At the age of 30, around 7% of HZ patients are affected by PHN, at the age of 50 it is around 12%, and at the age of 70 around 18% [14]. In post-herpetic neuralgia, the neuralgic pain can persist for several weeks, months or even years after the rash has subsided. PHN pain is limited to the respective dermatome and is typically described as burning or stabbing. Pain episodes can vary greatly from person to person, and in some cases they can have a significant impact on quality of life [15]. Herpes zoster ophthalmicus is a consequence of reactivation of the varicella zoster virus in the trigeminal ganglion and is a particularly serious complication. It can threaten eyesight and requires urgent antiviral treatment [16]. More rarely, other ophthalmologic, dermatologic, neurologic, visceral or vascular complications may occur [8]. |
Catch-up vaccination is recommended if the basic vaccination has been missed
Since January 1, 2023, the EKIF and the FOPH have recommended vaccination against varicella with two doses as the basic vaccination against chickenpox (varicella) for all infants aged 9 and 12 months in Switzerland [2,3]. Vaccination should preferably be carried out with a combined, quadrivalent MMRV vaccine that protects against four diseases: Measles, mumps, rubella and varicella [3]. As the risk of disease complications is increased in adults and infection should be prevented in all persons who are not yet immune, all children, adolescents and adults aged 13 months to 39 years who have not yet contracted varicella and who have not yet received a total of two vaccine doses are recommended to be revaccinated (1 or 2 doses) against chickenpox (or MMRV) [3]. If there is uncertainty about a previous chickenpox infection, IgG antibodies can be determined to clarify the immune status [3]. The costs of both the recommended basic vaccination and the booster vaccination using individual varicella vaccines are covered by compulsory health insurance (basic insurance) [3].
Shingrix® protects people over 65 and other risk groups against herpes zoster
Vaccination against herpes zoster with the adjuvanted subunit vaccine (Shingrix®) has been recommended in Switzerland since 2022 for healthy people aged 65 and over and for patients with immunodeficiency aged 50 and over or with severe immunodeficiency aged 18 and over [3,7]. Shingrix® is a recombinant, AS01B-adjuvanted subunit vaccine [7,8]. One dose (0.5 ml) contains 50 μg of the varicella zoster virus glycoprotein E (gE) antigen, which is produced using recombinant DNA technology. The adjuvant AS01B contains 50 μg of the plant extract Quillaja saponaria Molina and 50 μg of a monophosphoryllipid A (MPL) from Salmonella minnesota.
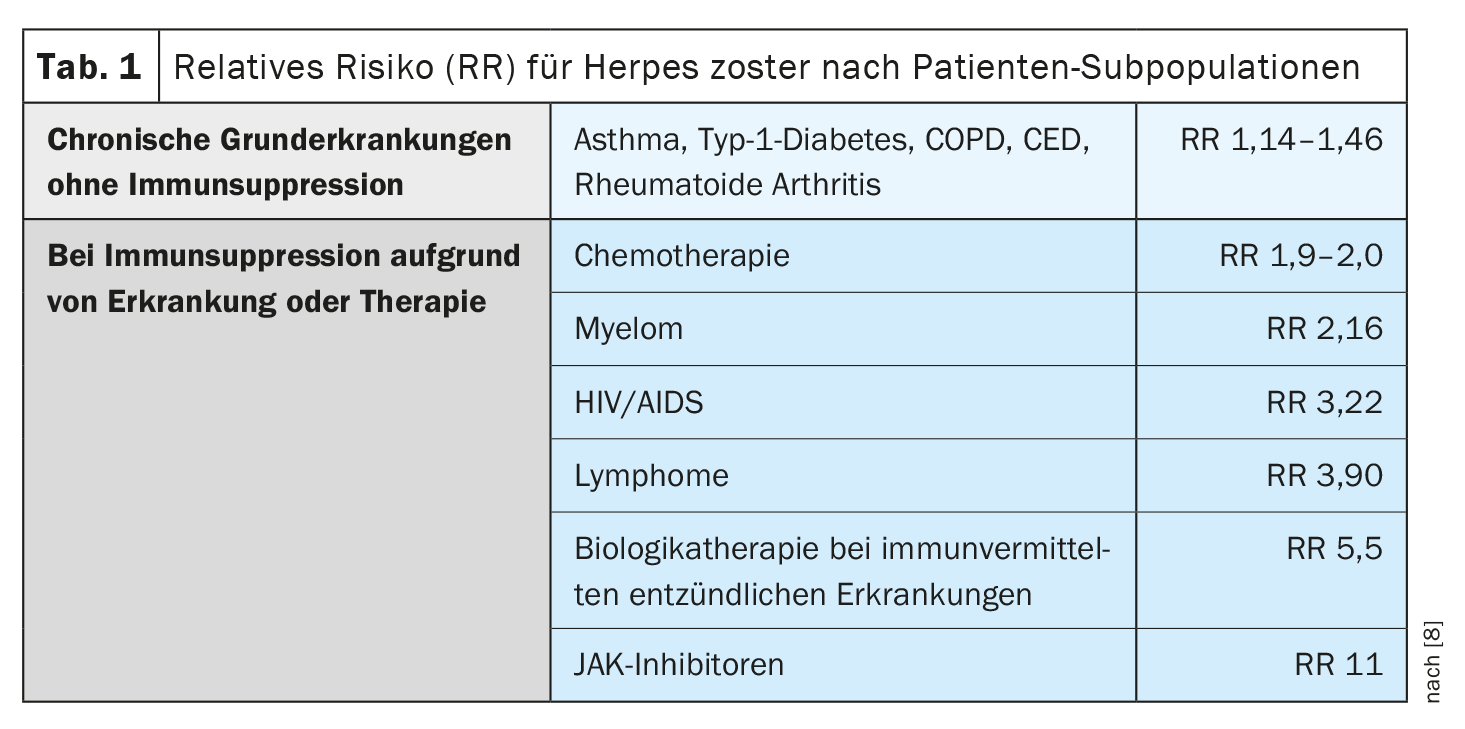
In many chronic underlying diseases without immunosuppression, the risk of herpes zoster is only slightly increased, but in the case of immunosuppression due to disease or therapy, it is sometimes massively higher compared to age-matched healthy individuals (Table 1) . [8]. In a meta-analysis of the risk of herpes zoster in immunocompromised individuals in the USA, the annual incidence was highest for hematologic malignancies and after hematopoietic stem cell transplantation, followed by organ transplantation [9–11].
Two doses of Shingrix® are required at least two months apart. Immunogenicity analyses showed that 2 doses of Shingrix® induce a >4-fold increase in the cellular immune response compared to 1 dose [5]. Two doses are therefore necessary for good immunogenicity, even after Zostavax® and even with increased reactogenicity [5]. For people with immunodeficiency, an interval of four weeks may also be considered on an individual basis. In Switzerland, vaccination with the Shingrix® subunit vaccine is reimbursed by compulsory health insurance [3].
The previous recommendations from 2017 for the live attenuated vaccine (Zostavax®) only apply to people aged 65 to 79 years without immunodeficiency who prefer the live vaccine to the subunit vaccine. Vaccination with the live attenuated vaccine is not covered by compulsory health insurance [3,7].
“ZOE50” and “ZOE-70”: Effectiveness of Shingrix® in ≥50 and ≥70-year-olds respectively
The placebo-controlled clinical efficacy study “ZOE50” examined 15,411 participants aged ≥50 years during a mean follow-up period of 3.2 years [8,12]. Six participants in the vaccine group and 210 in the placebo group developed herpes zoster (incidence rate 0.3 vs. 9.1 per 1000 person-years). The efficacy of the vaccine against herpes zoster was 97.2% (95% CI; 93.7–99.0%; p<0.001) [8,12]. The effectiveness for all age groups was between 96.6% and 97.9% [8,12]. In the very similar “ZOE-70” study with a total of 13,900 participants (aged ≥70 years), HZ occurred during a mean follow-up of 3.7 years in 23 participants after Shingrix® versus 223 after placebo (0.9 vs. 9.2 per 1000 person-years) [8,13]. The efficacy against herpes zoster was 89.8% (95% CI; 84.2-93.7%; p<0.001) and was similar in participants aged 70 to 79 years (90.0%) and participants aged 80 years and over (89.1%) [13].
Congress: SGAIM Fall Congress
Literature:
- “Shingles”, www.usz.ch/krankheit/guertelrose,(last accessed 11/21/2023)
- BAG: Kommentar zu den Änderungen der KLV vom 28. November 2022 per 1. Januar 2023 AS 2022 840 vom 22. Dezember 2022
- «Windpocken & Gürtelrose», www.bag.admin.ch/bag/de/home/krankheiten/krankheiten-im-ueberblick/windpocken.html#-1259201142, (last accessed 21.11.2023)
- Schiffner-Rohe J, et al.: Herpes zoster in Deutschland. Eine retrospektive Analyse von GKV-Daten. [Herpes zoster in Germany. A retrospective analyse of SHL data]. MMW Fortschr Med 2010 Jan;151 Suppl 4: 193–197.
- «Wie ein Impfstoff der Gürtelrose vorbeugen kann», Dr. med. Daniel Desgrandchamps, Satelliten Symposium GSK, SGAIM Herbstkongress, 21.–22.09.2023.
- “Shingles (herpes zoster) “. www.infovac.ch/de/impfunge/nach-krankheiten-geordnet/guertelrose-herpes-zoster,(last accessed 11/21/2023)
- Swissmedic: Arzneimittelinformationen,
www.swissmedicinfo.ch, (last accessed 21.11.2023) - BAG-Bulletin 47/2021, issue of November 22, 2022.
- McKay SL, et al.: Herpes Zoster Risk in Immunocompromised Adults in the United States: A Systematic Review. Clinical infectious diseases an official publication of the Infectious Diseases Society of America 2020; 71(7): e125–e134.
- Chen H-H, et al.: Risk of herpes zoster in patients with systemic lupus erythematosus: a three-year follow-up study using a nationwide population-based cohort. Clinics (Sao Paulo, Brazil) 2011; 66(7): 1177–1182.
- Bastidas A, et al.: Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster After Autologous Stem Cell Transplantation: A Randomized Clinical Trial. JAMA 2019; 322(2): 123–133.
- Lal H, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. The New England Journal of Medicine 2015; 372(22): 2087–2096.
- Cunningham AL, et al.: Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. The New England Journal of Medicine 2016; 375(11): 1019–1032.
- Hillebrand K, et al.: Incidence of herpes zoster and its complications in Germany, 2005–2009. The Journal of infection 2015; 70(2): 178–186.
- Johnson RW, et al.: The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC medicine 2010; 8: 37.
- Liesegang T: Herpes Zoster Ophthalmicus: Natural History, Risk Factors, Clinical Prevention and Morbidity. Ophthalmology 2008; 115(2); suppl): S3–S12.
HAUSARZT PRAXIS 2023; 18(12): 24–25