Two types of T-cell-based therapy are available for refractory or relapsing diffuse large B-cell lymphoma (DLBCL): CAR T cells and bispecific antibodies. While CAR T cells have revolutionized individualized medicine to a certain extent, generically available bispecific antibodies also offer advantages. The rapidly available antibody treatment has now confirmed its long-lasting efficacy profile in the 2-year follow-up of the EPCORE NHL-1 study.
DLBCL is the most common form of heterogeneous, aggressive non-Hodgkin’s lymphoma. While patients who respond to first-line therapy have a good chance of cure or survival, refractory and relapsed (R/R) patients who are not eligible for a stem cell transplant have a poor prognosis of only around 6.3 months on average [1]. For aggressive R/R DLBCL, various T-cell-based therapeutic approaches are now available after two systemic pre-treatments [1].
CAR T cells and bispecific antibodies
CAR T-cell therapy uses genetically modified T-cells to recognize overexpressed surface antigens on cancer cells and attack them specifically. This technology is used in specialized centers and has revolutionized the therapeutic landscape in various haemato-oncological indications, including DLBCL. It is individually tailored to the patient and leads to a very good response in patients [2, 3]. Possible side effects of CAR T-cell treatment that should be given special attention are cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) [2]. If patients are not eligible for CAR T-cell therapy, e.g. because a rapidly progressing disease makes the waiting period impossible, or CAR T-cell therapy has already been attempted, the bispecific antibody epcoritamab can also be used for R/R DBCLC after systemic pretreatment in at least two lines of therapy. This generic antibody binds both to an epitope of CD20, which is found in most B-cells of a B-cell lymphoma, and to CD3, which is expressed on T-cells. The simultaneous binding of CD20-expressing tumor cells and CD3-expressing endogenous T cells mediates specific T cell activation [4]. In addition to pyrexia and neutropenia, CRS is also considered a potentially serious side effect of this treatment option [1].
Effective alternative for DLBCL patients
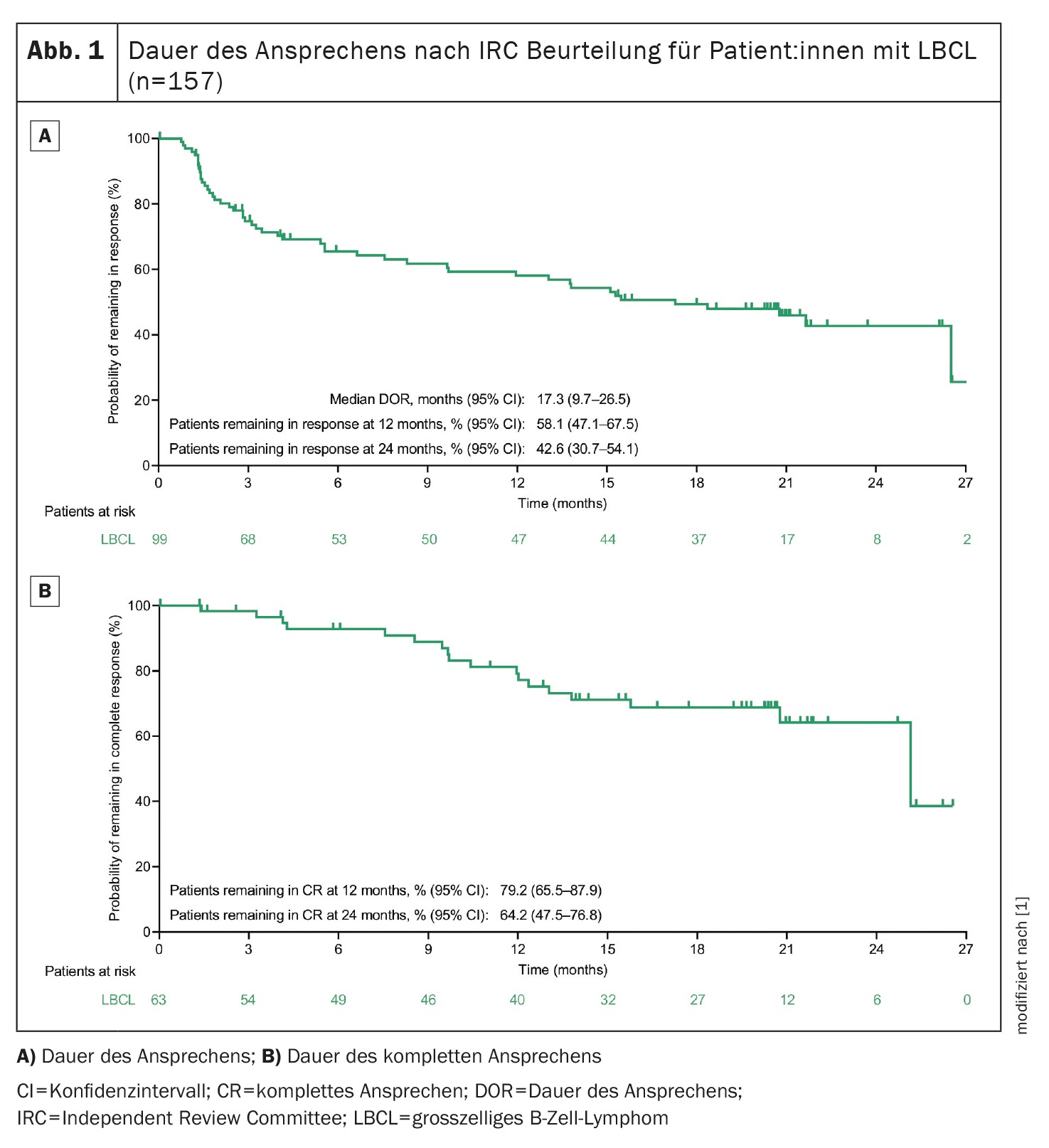
The 2-year follow-up of the ongoing phase 1/2 EPCORE NHL-1 study, which is investigating the bispecific antibody epcoritamab in R/R (D)LBCL patients, has just been published [1]. A total of 157 (D)LBCL patients undergoing epcoritamab monotherapy were included and the primary analysis had already shown an overall response rate (ORR) of 63.1% and a complete response (CR rate) of 38.9%. In addition, 45.8% of patients evaluable for minimal residual disease (MRD) achieved MRD negativity [5]. After a median follow-up of 25.1 months (95% CI, 24.0-26.0), 63.1% of patients responded to treatment (ORR, n/N=99/157; 95% CI, 55.0-70.6) and 40.1%** (n/N=63/157; 95% CI, 32.4-48.2) achieved a CR in the current evaluation of the study. The estimated progression-free survival (PFS) rate at 2 years was 27.8% and the estimated overall survival (OS) rate was 44.6% [1]. The median follow-up for duration of response (DOR) was 20.8 months (95% CI, 20.1-21.2) and the median DOR was 17.3 months (95% CI, 9.7-26.5) (Fig. 1) [1]. An estimated 64.2% of patients with CR also showed this after 2 years. The CR rate was consistent with the overall population regardless of subgroups (e.g. age, ECOG status, number of prior treatments, prior CAR T-cell therapy, refractory to last anti-CD20 therapy, de novo vs. transformed DLBCL) [1]. MRD negativity was detected in 54 of the 119 MRD evaluable patients (45.4%; 95% CI, 36.2-54.8). CR and MDR negativity were associated with long-term PFS and OS [1].
** per Independent Review Committee (IRC) based on the Lugano criteria
The most common adverse events with the bispecific antibody epcoritamab were CRS (51.0%), pyrexia (24.8%), fatigue (24.2%) and neutropenia (23.6%). Fatigue was more common during the first 8 weeks of treatment than in later phases, while the occurrence of neutropenia was consistent over the treatment period [1]. Treatment-related adverse events leading to discontinuation of treatment occurred in 23 patients (14.6%). No new cases of ICANS, CRS or clinical tumor lysis syndrome (TLS) were reported during the follow-up period [1].
Conclusion
Although CAR T-cell therapy has revolutionized the treatment landscape for some hemato-oncology indications, there are patients who are not eligible for this therapy or who show progression after personalized treatment. DLBCL patients may benefit from the tolerable, generically available option of the bispecific antibody epcoritamab [1,3,4]. The long-lasting response in R/R DLBCL patients with poor prognosis was confirmed in the recently published 2-year follow-up of the EPCORE-NHL-1 study [1].
Literature:
- Thieblemont C, et al: Epcoritamab in relapsed/refractory large B-cell lymphoma: 2-year follow-up from the pivotal EPCORE NHL-1 trial. Leukemia 2024.
- Chohan KL, Siegler EL, Kenderian SS: CAR-T Cell Therapy: the Efficacy and Toxicity Balance. Curr Hematol Malig Rep 2023; 18(2): 9-18.
- Neelapu SS, et al: Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 2023; 141(19): 2307-2315.
- Current information for healthcare professionals Tepkinly® (epcoritamab); www.swissmedicinfo.ch.
- Thieblemont C, et al: Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell-Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J Clin Oncol 2023; 41(12): 2238-2247.
InFo ONKOLOGIE & HÄMATOLOGIE 2024; 12(6): 28–29
Cover picture: Micrograph of a diffuse large B cell lymphoma, abbreviated DLBCL. Lymph node FNA specimen. Field stain. © Nephron, wikimedia