Takotsubo syndrome is an important differential diagnosis in patients with acute coronary syndrome and is associated with a significant risk of life-threatening complications. It predominantly affects postmenopausal women and often there is a precipitating event. The prognosis is comparable to that after an acute myocardial infarction.
Takotsubo syndrome is an important differential diagnosis in patients with acute coronary syndrome and is associated with a significant risk of life-threatening complications. The essential aspects of the disease are summarized in this article.
Takotsubo syndrome (TTS) is characterized by acute but completely reversible left ventricular dysfunction that is not due to significant coronary artery stenosis [1]. Characteristic is the striking distribution of wall motion abnormalities with regional “ballooning” of the left ventricle [1–3]. The disease was first described in Japan in 1990. Case series from Europe and North America did not follow until more than ten years after the initial description. As a result, awareness and interest in TTS increased, resulting in a significant increase in scientific papers in recent years. Although the term TTS is now established, earlier common terms such as “Takotsubo cardiomyopathy,” “broken heart syndrome,” “apical ballooning syndrome,” or “stress cardiomyopathy” can still be found in the literature.
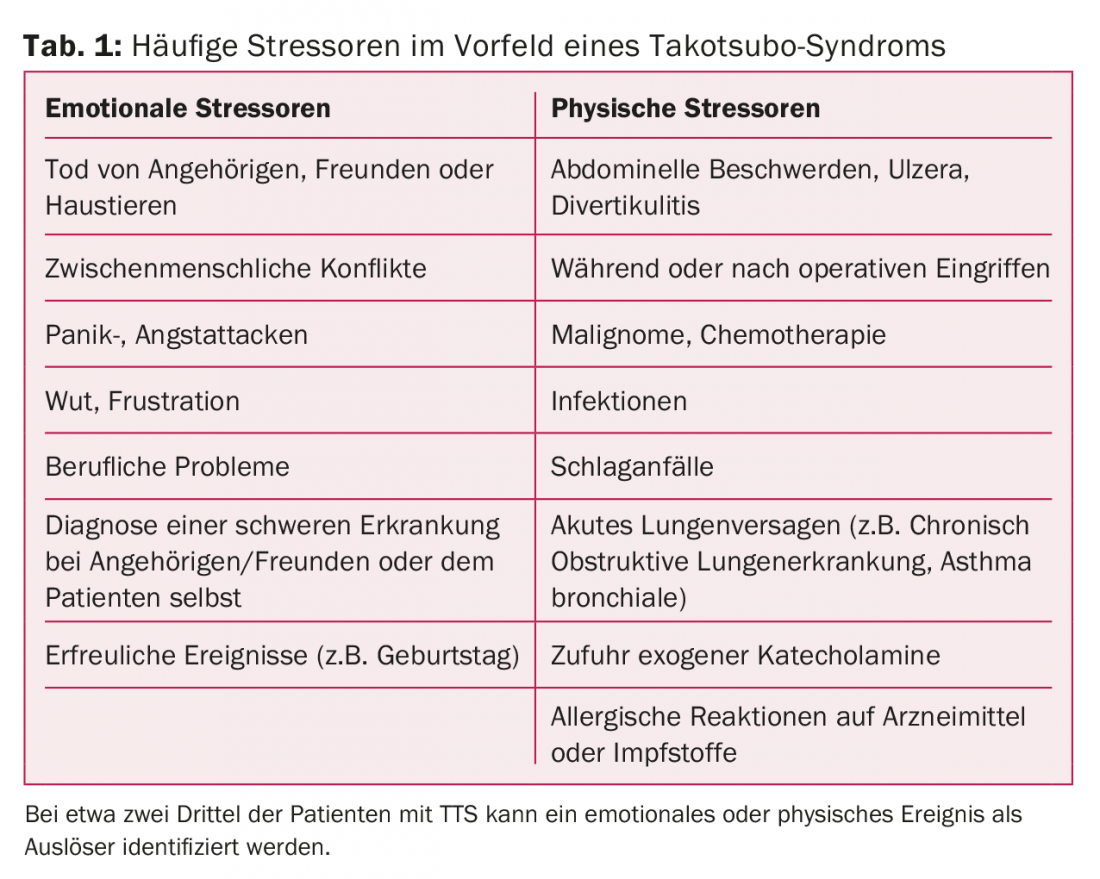
Patients with TTS typically present with angina pectoris symptoms and/or dyspnea and are thus indistinguishable from patients with acute coronary syndrome (ACS) on the basis of symptoms [1,2]. It is estimated that approximately 2% of all patients whose initial clinical symptoms are suggestive of ACS have TTS. Women are affected much more frequently than men [1–3]. In some collectives, the proportion of women is over 90%, with postmenopausal women in particular at increased risk for TTS. A distinctive feature of TTS is a precipitating event prior to the episode, which can be found in about two-thirds of patients [1–3]. This stressor can be both physical and emotional, and even pleasurable events can result in TTS (Table 1).
Diagnostic clarification
Because of the clinical presentation of patients with TTS, recording an ECG is the first step in the differential diagnostic workup. Here, similar to patients with ACS, ST-segment elevations, ST-segment depressions, or T-negativations are possible [1]. However, the changes may be nonspecific, and an unremarkable ECG does not rule out TTS. In some collectives, patients showed periodic ECG changes with initial ST-segment elevations followed by T-negativations and QT prolongation in the subacute phase.

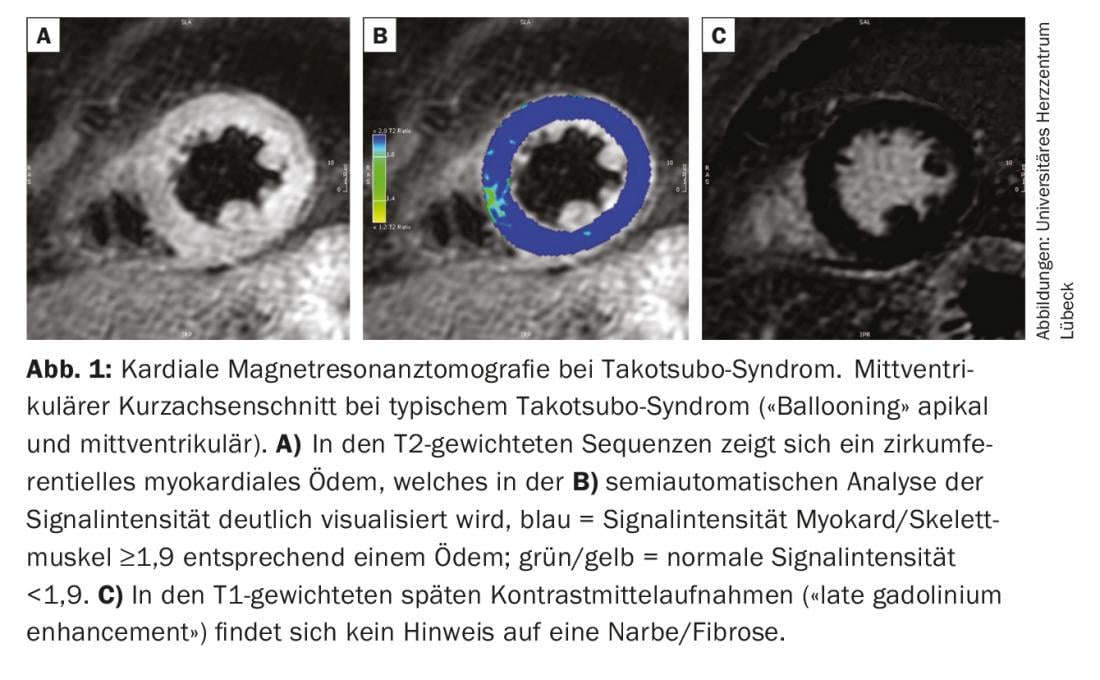
Cardiac biomarkers (troponin, creatine kinase) may be moderately elevated in the setting of TTS, with maximal levels usually much lower than in patients with acute myocardial infarction [1]. In contrast, TTS patients often have significantly higher concentrations of natriuretic peptides. Nevertheless, even on the basis of laboratory chemistry parameters, it is not possible to reliably distinguish between a TTS and an ACS. For this, it is essential to perform coronary angiography. In contrast to ACS, coronary stenoses are not found in TTS patients, which may explain the extent of kinetics abnormalities [1]. The diagnosis is usually guided by visualization of the characteristic left ventricular wall motion abnormalities by laevocardiography or echocardiography. The criteria required to confirm TTS were established in a statement by the European Society of Cardiology and are summarized in Table 2 [1]. Because reversibility of left ventricular dysfunction is explicitly part of the definition, the diagnosis cannot be definitively made until normal systolic function is documented during follow-up. However, this may take a few weeks. Cardiac magnetic resonance imaging provides important additional information to confirm the diagnosis already in the acute phase. In the case of TTS, this often reveals regional myocardial edema with signs of diffuse inflammation without evidence of irreversible damage in the form of fibrosis or necrosis (Fig. 1) [2]. Magnetic resonance imaging thus provides important differential diagnostic information to differentiate myocardial infarction with spontaneous lysis or myocarditis and should therefore be performed in all patients with questionable TTS when the opportunity exists.
Forms of Takotsubo syndrome
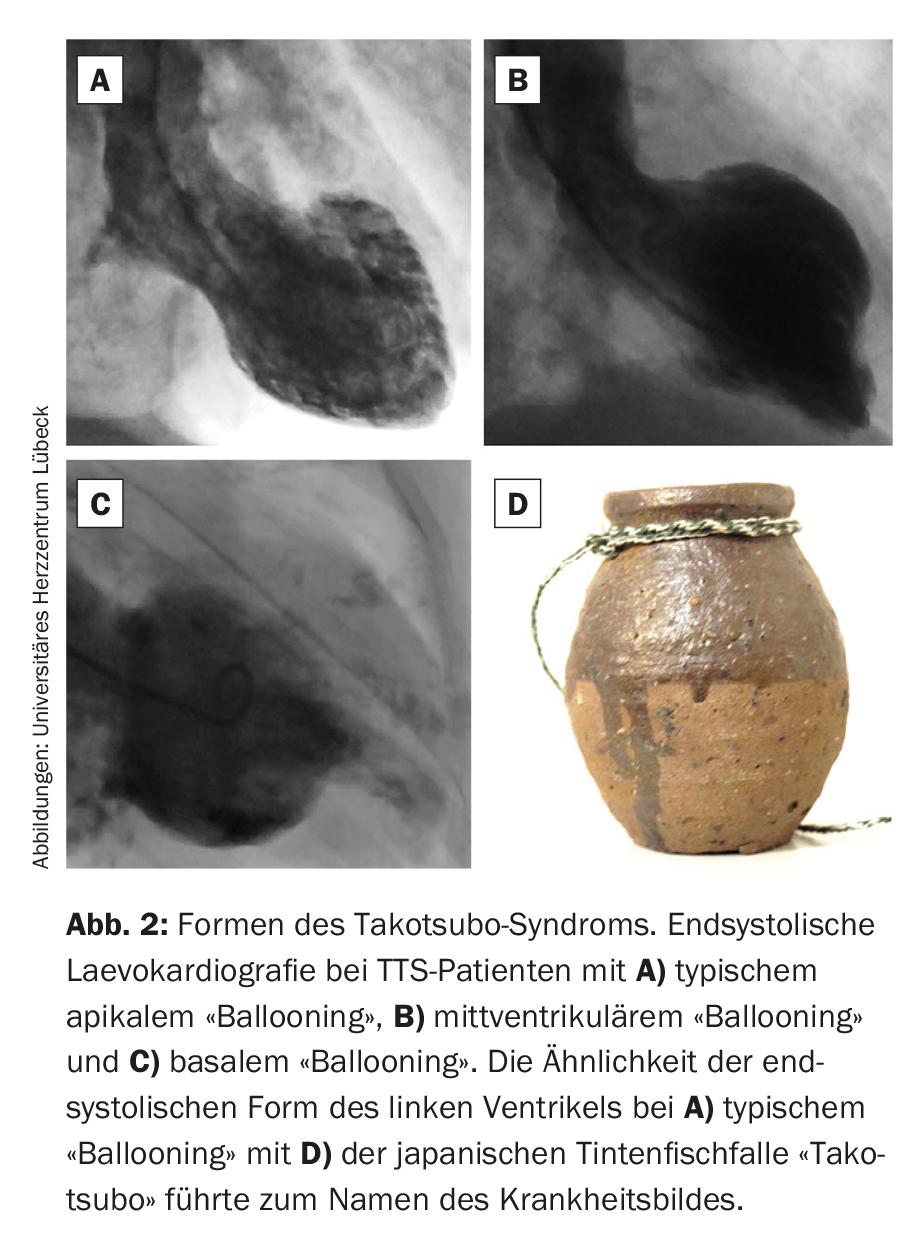
Based on the distribution of kinetic disturbances of the left ventricle, three main forms of TTS are distinguished (fig. 2). The classic and, with approximately 75%, most frequent form is characterized by basal hypercontractility with midventricular and apical akinesia (typical apical “ballooning”). The endsystolic contour reminded the first describer of a Japanese octopus trap in the shape of a jar with a round bottom and a short neck (“takotsubo”). This led to the name of the disease. In atypical forms of TTS, either the midventricular (midventricular “ballooning,” approximately 20% of TTS cases) or basal portions (basal “ballooning,” approximately 5% of TTS cases) are hypo- or akinetic. In addition, a very rare focal form of TTS has recently been described [3]. However, this differs fundamentally from the established forms because of its non-circular extension and absolutely requires accurate differential diagnostic workup by magnetic resonance imaging. In addition to the left ventricle, the right ventricle is also affected by kinetic dysfunction in approximately one third of patients with TTS, and isolated right ventricular forms have also been described [1,2].
Pathophysiology
Regarding the pathogenesis of TTS, numerous theories have been proposed. However, despite increasing scientific efforts, the underlying mechanisms have not yet been definitively clarified. Observation of precipitating stressors in a large number of the patients led to the assumption that increased sympathetic nervous system activity and elevated catecholamine levels may play a leading role in the pathophysiology [4]. Support for this theory was provided by the demonstration of increased plasma catecholamine levels in patients with TTS, by the observation of left ventricular dysfunction after external administration of catecholamines as well as in patients with catecholamine-producing tumors, and by morphological changes in histological studies that were comparable to the known cardiotoxic effects of catecholamines. However, after triggering events and elevated catecholamine levels were not detected in all TTS patients, doubts also arose about this pathophysiological model. Furthermore, systemically elevated catecholamine levels do not provide an explanation for the striking distribution of wall motion abnormalities with occasional right ventricular involvement.
An alternative concept considers the leading cause of TTS to be coronary microcirculatory disturbances, which have been demonstrated in several studies of TTS patients using a variety of methods. Accordingly, while the occurrence of microcirculatory dysfunction in the setting of TTS is largely uncontroversial, it remains unclear whether the decreased coronary flow is causative or a secondary phenomenon due to left ventricular dysfunction. Recent studies also indicate a possible genetic predisposition for the occurrence of TTS [5]. However, further studies in large patient collectives are needed for this purpose. Because of its predominant occurrence in postmenopausal women, the possible influence of estrogens is also the subject of scientific investigation. Other postulated pathophysiological concepts such as left ventricular outflow tract obstruction, coronary vasospasm, muscle bridging, or plaque rupture with spontaneous thrombus lysis have since been refuted in studies or appear highly unlikely due to the low prevalence in TTS patients [6].
Therapy
Since there are no evidence-based recommendations regarding therapy in patients with TTS, treatment decisions are made on an individual basis. In the acute phase of the disease, the focus is on continuous cardiovascular monitoring and management of any complications [1]. In most cases, heart failure therapy is initiated according to the clinical symptoms (ACE inhibitors, beta blockers, diuretics if necessary). During the course, normalization of left ventricular function and ECG changes are documented by regular checks. Constant adjustment of drug therapy to the current clinic is necessary.
Forecast
Because of the complete regression of left ventricular kinetic dysfunction, patients after TTS were originally considered to have a good prognosis. However, this assumption has been refuted by several studies in recent years. These show similar or even higher short- and long-term mortality rates after TTS compared with patients with acute myocardial infarction [3,7,8]. Serious complications can occur, especially during the acute phase of the disease. These include, but are not limited to, severe heart failure with pulmonary edema to cardiogenic shock or life-threatening cardiac arrhythmias [9,10]. The risk of arrhythmias should not be underestimated even in the subacute phase until complete normalization of left ventricular function and ECG changes (especially a possibly prolonged QT time). In the long-term course, noncardiovascular concomitant diseases (e.g., malignancies) also contribute decisively to the prognosis [11]. Numerous risk factors for adverse events have been identified for prognostic assessment [1,7]. These include age, male sex, physical stressors, extent of left ventricular dysfunction, and right ventricular involvement. The recurrence rate after an episode of TTS is between 5-10%. Interestingly, in the case of recurrent TTS episodes, a patient may show different “ballooning” forms.
Take-Home Messages
- TTS is an important differential diagnosis in patients with suspected acute coronary syndrome without causative coronary stenosis and is diagnosed based on specific criteria.
- It predominantly affects postmenopausal women and often there is a precipitating event.
- Cardiac magnetic resonance imaging provides valuable additional information to confirm the diagnosis.
- Increased sympathetic activity and elevated catecholamine levels, coronary microcirculatory dysfunction, and genetic predisposition appear to play a pathophysiological role. However, the exact disease mechanisms have not been definitively clarified.
- Despite complete normalization of left ventricular function, the prognosis after TTS is comparable to that after acute myocardial infarction.
Literature:
- Lyon AR, et al: Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2016; 18: 8-27.
- Eitel I, et al: Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA 2011; 306: 277-286.
- Templin C, et al: Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med 2015; 373: 929-938.
- Wittstein IS, et al: Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352: 539-548.
- Eitel I, et al: Genome-wide association study in takotsubo syndrome – Preliminary results and future directions. Int J Cardiol 2017; 236: 335-339.
- Eitel I, et al: Optical Coherence Tomography to Evaluate Plaque Burden and Morphology in Patients With Takotsubo Syndrome. J Am Heart Assoc 2016; 5(12). pii: e004474.
- Stiermaier T, et al: Long-term excess mortality in takotsubo cardiomyopathy: predictors, causes and clinical consequences. Eur J Heart Fail 2016; 18: 650-656.
- Redfors B, et al: Mortality in takotsubo syndrome is similar to mortality in myocardial infarction – A report from the SWEDEHEART registry. Int J Cardiol 2015; 185: 282-289.
- Stiermaier T, et al: Prevalence and Clinical Significance of Life-Threatening Arrhythmias in Takotsubo Cardiomyopathy. J Am Coll Cardiol 2015; 65: 2148-2150.
- Stiermaier T, et al: Incidence, determinants and prognostic relevance of cardiogenic shock in patients with Takotsubo cardiomyopathy. Eur Heart J Acute Cardiovasc Care 2016; 5: 489-496.
- Moller C, et al: Prevalence and long-term prognostic impact of malignancy in patients with Takotsubo syndrome. Eur J Heart Fail 2017. doi: 10.1002/ejhf.868. [Epub ahead of print].
CARDIOVASC 2018; 17(1): 4-7











