Patients with non-resectable metastatic melanoma with BRAFV600 mutation should first be evaluated for eligibility for immunotherapy. In addition to checkpoint inhibition, however, targeted therapy with BRAF-MEK inhibitors is now an established treatment option. Three combinations of BRAF and MEK inhibitors are currently available in Switzerland. In terms of both efficacy and tolerability, combined use has been shown to be superior to monotherapy.
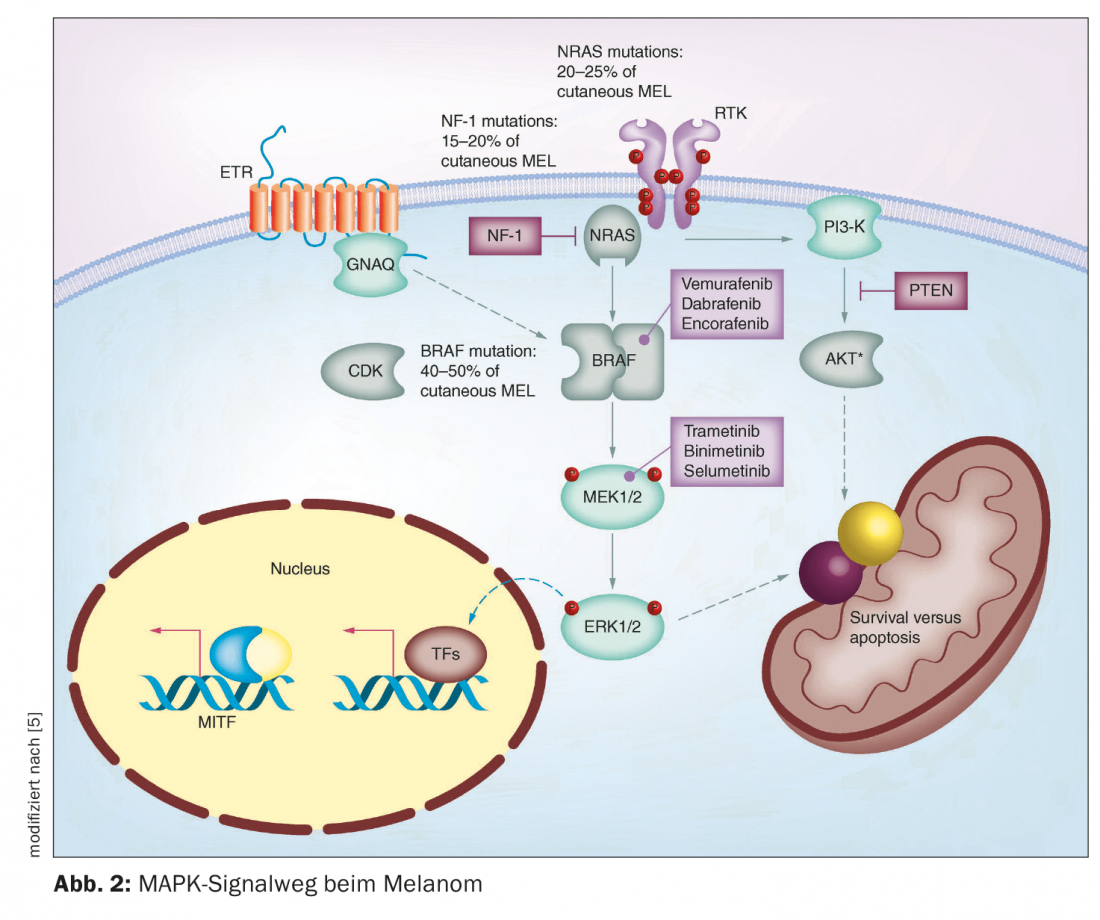
In recent years, several breakthroughs have been achieved in the field of malignant melanoma therapy. Until about the mid-1990s, only chemotherapies were available. These inhibit cell growth and cell division relatively unspecifically and thus sometimes cause considerable side effects [1]. The market approval of immuno-oncology and targeted therapies have significantly changed treatment options for unresectable metastatic melanoma. In melanoma patients with non-resectable metastases, the option of immunotherapy with checkpoint inhibitors should be considered first. If patients are eligible for immunotherapy, the current ESMO guidelines recommend immunotherapy with checkpoint inhibitors as first-line therapy (Fig. 1) [2]. However, in BRAF-mutated non-resectable metastatic melanoma, the use of targeted therapy with BRAF/MEK inhibition is also possible. Both checkpoint inhibition and targeted therapy have advantages and disadvantages [3]. Prof. Dr. med. Reinhard Dummer, Deput. Clinic Director Dermatology Clinic and Head Skin Tumor Center, University Hospital Zurich, summarized important facts about BRAF/MEK inhibition on the occasion of this year’s Zurich Dermatology Training Days [4]. This targeted combination therapy inhibits the “mitogen-activated protein kinase” (MAPK) signaling pathway that is continuously activated in BRAFV600 mutation (Fig. 2) [5].
Mutation analysis for the detection of BRAFV600E/K mutation status.
BRAFV600E/K mutation status is a prerequisite for the use of BRAF/MEK inhibitors. BRAF is the most common mutation in melanoma, typically occurring in younger melanoma patients. Often it is patients who have many nevi. In particular, smaller and acquired melanocytic nevi have a very high frequency for BRAF mutations, Prof. Dummer said. In locally advanced or metastatic stage III and IV melanoma, molecular pathology diagnostics should be performed to determine BRAF status. [6]. BRAF mutation analysis can be performed on biopsy material or punches of the affected skin areas as well as on fixed and paraffin-embedded tumor material [7]. PCR technique can be used to amplify the relevant regions of BRAF gene from genomic DNA and analyzed by DNA sequencing [8]. According to the Genomic Classification of Cutaneous Melanoma, a division can be made into four subtypes, which have different prevalences: BRAF mutation melanomas (50%), N-Ras, K-Ras, or H-Ras mutations (25%), NF1 mutations (15%), and triple wild-type melanomas (10%) [9]. The BRAF gene encodes the serine-threonine protein kinase BRAF. This plays a role in cell growth regulation via the MAP kinase and Ras-Raf signaling pathways. A mutation with exchange of the amino acid valine at position V600 for glutamine (V600E) or for lysine (V600K) in the BRAF protein constitutively upregulates BRAF kinase activity [7]. As a result, a signaling cascade is permanently activated, leading to uncontrolled cell growth.
Which BRAF/MEK inhibitor therapies are available in Switzerland?
Before the combinations of BRAF and MEK inhibitors came on the market, clinical trials first tested BRAF inhibitors as monotherapy compared to chemotherapy. The kinase inhibitors were far superior, with a hazard ratio (HR) of 0.37 in favor of dabrafenib and 0.38 in favor of vemurafenib. “That means more than a 60% improvement in progression-free interval with these inhibitors. That’s a huge difference,” Prof. Dummer explained. A next step was to combine BRAF and MEK inhibitors in clinical trials. Additional administration of a MEK inhibitor to a BRAF inhibitor should prevent early development of resistance by reactivating the MAPK cascade and paradoxically activating these pathways in healthy cells without mutation of the BRAF gene [10].
Clinical trials were conducted, in each case according to the principle of monotherapy vs. combination therapy, with a clear advantage always being shown in favor of combined use. BRAF-i/MEK-i combination therapies have similar or better tolerability than monotherapy with BRAF-i, the speaker explained [4]. Currently, three BRAF-i/MEK-i combinations are available in Switzerland: Dabrafenib/Trametinib, Vemurafenib/Cobimetinib, Encorafenib/Binimetinib. “For triple treatment, we primarily use vemurafenib/cobimetinib. For patients who become progressive directly after immunotherapy, encorafenib/binimetinib and in the adjuvant setting we have very good data for dabrafenib/trametinib”, explains Prof. Dummer and adds: “Also for brain metastases we have very good results for the combination dabrafenib/trametinib” [4].
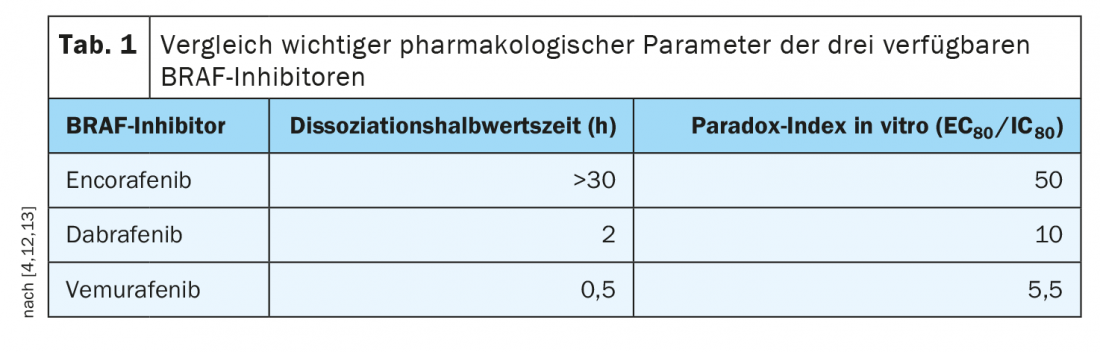

Encorafenib exhibits advantageous pharmacological properties
The tolerability profiles of the various combination therapies differ somewhat; this should be discussed with patients. For example, there is evidence that encorafenib/binimetinib reduces the risk of pyrexia (fever) and photosensitivity, and there is good empirical evidence for long-term use. While with vemurafenib, pathway inhibition is very closely correlated with serum levels, with encorafenib, serum levels may decrease but inhibition persists. This extends over more than 30 hours. This particularly long dissociation half-life of encorafenib (Tab. 1), means a stable blockage of the signaling pathway and this is clinically relevant, emphasizes Prof. Dummer [4]. In addition, the values in the Paradox index, which is a measure of side effects on healthy cells (i.e., those without mutation of the BRAF gene), were favorable for encorafenib, the speaker said. The combined use of encorafenib with the MEK inhibitor binimetinib allows a dose increase of encorafenib and a further increase in efficacy. “Because the MEK inhibitor still improves tolerability, you can increase the dose of encorafenib in combination with binimetinib,” Prof. Dummer elaborates [4]. This was investigated in the three-arm phase III COLUMBUS trial [11]. This compared encorafenib 450 mg (1×/d) plus binimetinib 45 mg (2×/d) with encorafenib 300 mg (1×/d) plus vemurafenib 960 mg (2×/d). Included were a total of 577 patients with newly diagnosed advanced/metastatic BRAFV600-mutated melanoma or progression after first-line immunotherapy. The targeted combination therapy of encorafenib plus binimetinib (COMBO450) proved significantly superior to monotherapy with vemurafenib and encorafenib, respectively, with a median PFS of 14.9 months. With regard to side effect risks, it is now known that MEK-i-associated retinopathy is completely regressive and there is no permanent damage to the retina even with long-term use, Prof. Dummer said. If patients experience corresponding side effects, these can be treated locally with anti-inflammatory agents and usually disappear after a short time.
Congress: Zurich Dermatological Training Days
Literature:
- “Precision oncology – Evolution of drug-based cancer therapy to personalized cancer therapy,” Thomas Kubin, M.D., www.kliniken-suedostbayern.de/files/PDF-Dokumente/oz/Newsletter_OnkoKrebszentr_3_2021_web.pdf, (last accessed Sept. 14, 2022).
- Michielin O, et al: Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol 2019; 30(12): 1884-1901.
- Reschke R, et al: Therapy comprehension and health-related quality of life in patients with stage III/IV melanoma treated with new adjuvant therapies. J Dtsch Dermatol Ges 2021; 19(2): 215-222.
- “What should dermatologists know about BRAF and MEK inhibitors in melanoma?”, Prof. Reinhard Dummer, MD, Zurich Dermatology Education Days, 6/16-17/2022.
- Koelblinger P, Dornbierer J, Dummer R: A review of binimetinib for the treatment of mutant cutaneous melanoma. Future Oncol 2017; 13(20): 1755-1766.
- Hoffmann M, Özdemir B: Malignant melanoma : adjuvant therapy: state of the art, DERMATOLOGIE PRAXIS 2022; 32(1): 11-16.
- “Mutational analysis of malignant melanoma – the path to individualized therapy,” www.wisplinghoff.de/fileadmin/user_upload/Redakteure/Drucksachen/Laborinformationen/LabInfo_Mutationsanalyse_des_malignen_Melanoms_web.pdf, (last accessed Sept. 14, 2022).
- “Determination of BRAF Mutation Status,” www.ukaachen.de/fileadmin/files/institute/pathologie/Bestimmung_des_BRAF-Mutationsstatus_v2022.pdf, (last accessed Sept. 14, 2022).
- Akbani R, et al: Genomic Classification of Cutaneous Melanoma. Cell 2015; 161(7): 1681-1696.
- Hermann RM, Christiansen H: BRAF-mutated metastatic melanoma: initial data on long-term efficacy of targeted therapies. Radiation Oncol 2019; 195: 940-942.
- Dummer R, et al: Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018; 19(5): 603-615.
- Koelblinger P, Thuerigen O, Dummer R: Development of encorafenib for BRAF-mutated advanced melanoma. Curr Opin Oncol. 2018;30(2): 125-133.
- Adelmann C, et al: Comparative profiles of BRAF inhibitors: the paradox index as a predictor of clinical toxicity. Oncotarget 2016; 7: 30453-30460.
DERMATOLOGIE PRAXIS 2022; 32(5): 43-45