“It is more important to know which person has a disease than to know which disease a person has” – this sentence is attributed to Hippocrates, which means that the ancient Greek knew about the usefulness of personalized therapy already about 2400 years ago. The extent to which such an approach can also help in rheumatology to identify patients at risk for RA at an early stage and to treat them in a targeted manner is the subject of current studies.
The concept of “one size fits all” medicine, i.e. the standardized course of therapy irrespective of the peculiarities of the individual patient, naturally also always entails a relatively high proportion of (severe) side effects and non-responders. The effects of personalized medicine, based on molecular diagnostics (biomarker analysis) and biomarker-based stratification of patients, are intended to reduce these and increase the rate of responders.
The list of drugs for which genetic testing is mandatory in Germany extends over 29 pages on the website of the German Association of Research-Based Pharmaceutical Companies (vfa). Rheumatic diseases are not yet on this list. For Prof. Dr. Andrea Rubbert-Roth from the Clinic for Rheumatology at the Cantonal Hospital in St. Gallen, this is clear evidence that the topic of personalized medicine has not yet been able to establish itself in the field of rheumatology.
It is known that there is a genetic susceptibility to rheumatoid arthritis (RA) in addition to environmental factors such as smoking, dusts, and textile fibers. Already in 2007, a large data analysis with 14 000 patients as well as 3000 control subjects in 7 diseases showed that in the case of RA with HLA and PTPN22 two genes stand out. Prof. Rubbert-Roth recalled the hypothesis called Shared Epitopes (SE), according to which the HLA-DRB1 alleles associated with RA encode a common sequence of amino acids in the 3rd hypervariable region of the DRβ1 chain.
SE-positive patients benefit more
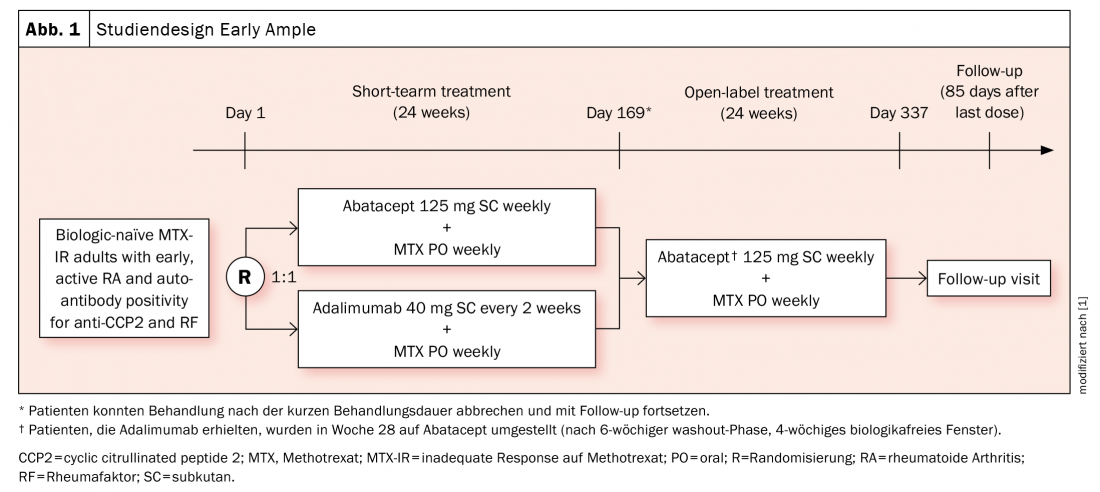
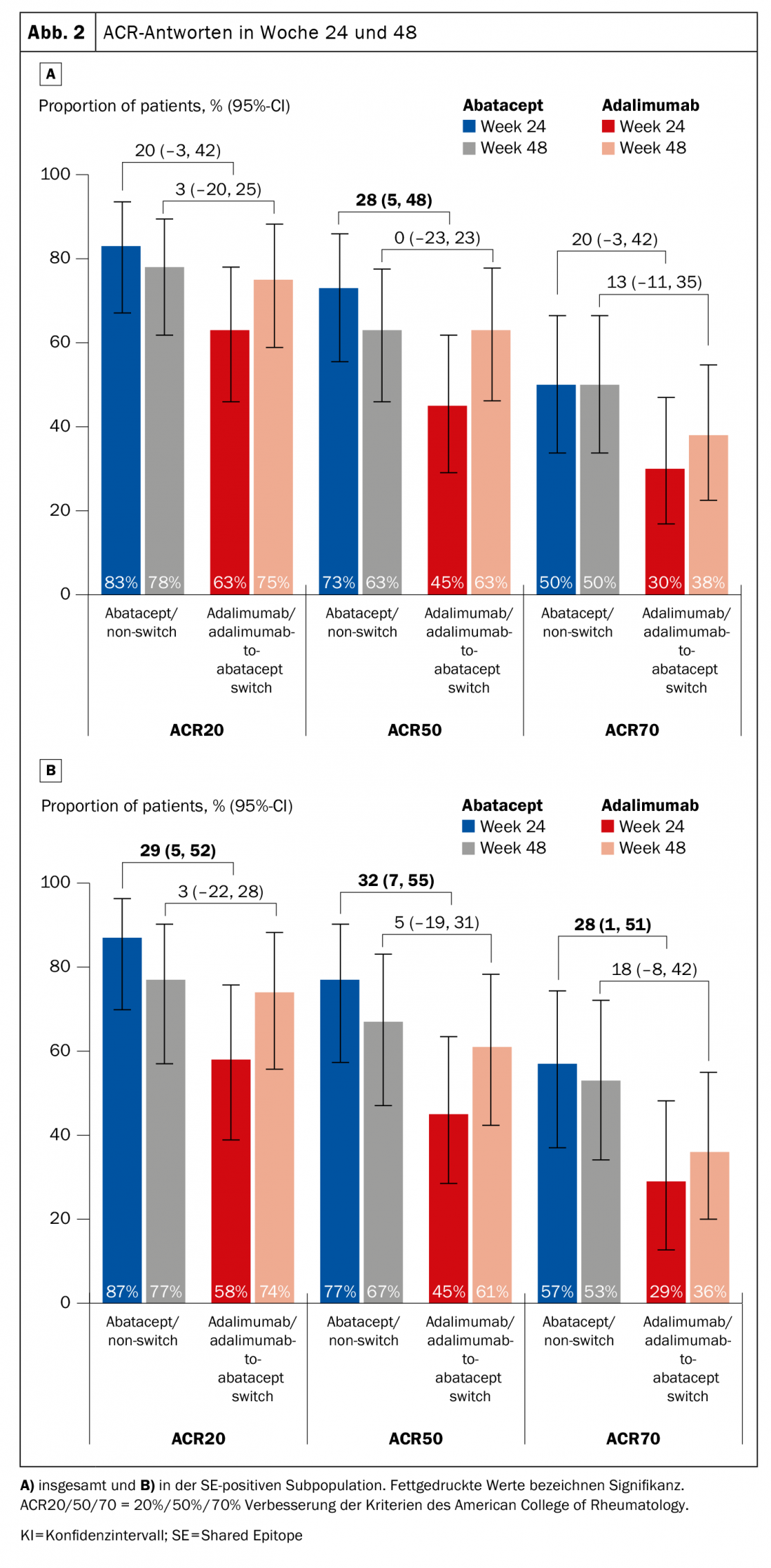
Previous evaluations have provided evidence that HLA-DRB1-positive patients respond better to abatacept (which does not mean that this cannot apply to other agents, the expert pointed out). The phase 4 Early Ample study [1], published in 2021, is based on this and divided patients with biologic-naïve early RA who had not responded adequately to methotrexate (MTX) into an abatacept (n=39, of which 30 were SE-positive) and an adalimumab arm (n=40, of which 31 were SE-positive) (Fig. 1) . Patients were required to be CCP2 and rheumatoid factor positive. Over 24 weeks, subjects were randomized to either ABA or ADA and subsequently all were switched to ABA. It was shown that the HLA-DRB1-positive patients already had significantly better chances of achieving an ACR20, -50, and -70 with abatacept after 24 and 48 weeks (Fig. 2A). The result was even stronger in favor of the SE+ subpopulation (Fig. 2B).
Can RA be prevented in patients at risk?
Patients who do not yet have clinically apparent synovitis but already have detectable CCP antibodies are known to be at increased risk of developing RA. Whether and how it is possible to prevent early development to RA in this group is the subject of the ARIAA study presented at the 2021 ACR Congress [2]. Inclusion criteria for this phase 2 study were ACPA positivity (+/- RF), arthralgias for at least 6 weeks, signs of inflammation of the dominant hand (synovitis, tenosynovitis, or osteitis) on MRI but no clinical swelling, and no previous therapy with steroids or DMARDs. Forty-nine patients so identified were treated with abatacept over a 6-month period versus placebo (n=49). This was followed by a 12-month follow-up period without treatment.
The primary endpoint, improvement in at least one MRI parameter (RAMRIS score), was met: there was significant improvement in 61.2% of patients in the abatacept arm (vs. 30.6% on placebo; p=0.0043). The verum group also performed significantly better on each of the secondary endpoints of premature study discontinuation and progression to clinical RA (discontinuation 14.3% vs. 42.9%; p=0.0032; progression 8.2% vs. 34.7%; p=0.0025). The benefit for abatacept compared to placebo is therefore clearly visible and Prof. Rubbert-Roth hopes to have the further results of the long-term follow-up presented at the upcoming EULAR. “If the results were confirmed over the follow-up period, it would be truly spectacular,” she concluded.
Take-Home Messages
- The implementation of new study concepts including a personalized strategy can also help to optimize the efficiency and safety of DMARD therapy in rheumatology.
- Proof-of-principle: Ideally, biomarkers would be readily accessible and easy to determine in the future.
- Can this concept be applied to other rheumatologic diseases? (e.g. interferon signature in SLE).
Literature:
- Rigby W, Buckner JH, Louis Bridges S, et al: HLA-DRB1 risk alleles for RA are associated with differential clinical responsiveness to abatacept and adalimumab: data from a head-to-head, randomized, single-blind study in autoantibody-positive early RA. Arthritis Res Ther 2021; 23: 245; doi: 10.1186/s13075-021-02607-7.
- Rech J, et al: ACR 2021 [#0455].
InFo PAIN & GERIATry 2022; 4(1-2): 20-21.