Current therapeutic strategies for inflammatory bowel disease (IBD) have reached a plateau in terms of response and/or remission rates achieved with a single therapeutic agent. Consequently, advanced combination therapy (ACT) has emerged as a new treatment concept. A group of researchers evaluated recent studies comparing ACTs with monotherapies.
Biologic therapies have become the standard of care for the treatment of moderate to severe active inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC). Despite these advances, current therapeutic approaches in IBD have stagnated in terms of response and/or remission rates. The existing arsenal of advanced therapies results in overall clinical remission rates of only about 50%. Furthermore, approximately 50% of patients who initially respond to biologic or small molecule therapy lose this response over time. Consequently, IBD patients who do not respond or have lost response to a particular biologic therapy must switch to a different molecule, reducing their prospects for long-term disease remission.
The novel approach of advanced combination therapy ( ACT) has emerged as a promising treatment strategy for IBD. It involves the use of two different targeted therapies, either biologic or small molecule, with the primary goal of overcoming the therapeutic plateau. Recently, data on ACT were obtained from the randomized controlled VEGA trial: Dr. Panu Wetwittayakhlang from the Department of Gastroenterology and Hepatology, Prince of Songkla University, Hat Yai, Thailand, and Dr. Peter L. Lakatos, Department of Gastroenterology and Hepatology, McGill University Health Center, Montreal, reviewed the current evidence (as of February 2024) on ACT and its impact on overcoming the therapeutic ceiling in the treatment of IBD [1].
Short-term combination of two biologics can help UC patients achieve better disease control
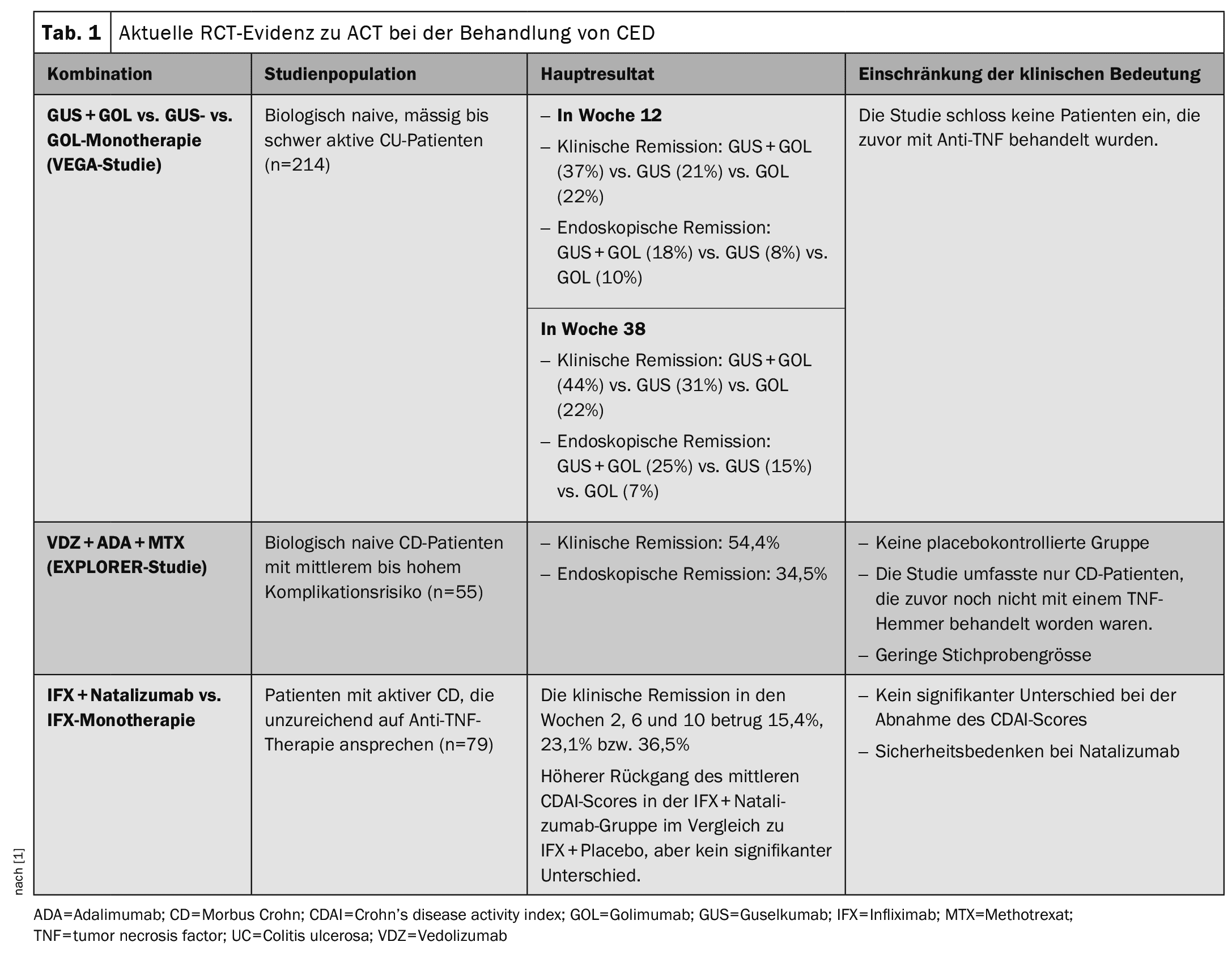
The phase 2a RCT VEGA investigated the efficacy of combined induction therapy with the selective interleukin (IL)-23 inhibitor guselkumab and the TNF-alpha inhibitor golimumab compared to guselkumab or golimumab monotherapy in patients with moderately to severely active CU. A total of 214 patients who had not responded to anti-TNF therapy and did not respond to or did not tolerate conventional therapy were randomly assigned to one of the following treatments:
- Guselkumab 200 mg i.v. in weeks 0, 4 and 8 (n=71),
- Golimumab 200 mg s.c. in week 0, then 100 mg s.c. in weeks 2, 6 and 10 (n=72)
- or a combination of 200 mg guselkumab i.v. plus 200 mg golimumab s.c. in week 0; 100 mg golimumab s.c. in weeks 2, 6 and 10 and 200 mg guselkumab i.v. in weeks 4 and 8 (n=71).
In the maintenance phase, patients in the combination arm were switched to monotherapy with guselkumab at the beginning of week 12.
In biologically untreated patients with CU, the study indicates a higher rate of clinical remission and endoscopic improvement in the combination arm (golimumab and guselkumab induction therapy) vs. the monotherapies with golimumab or guselkumab. However, the authors note that the VEGA study did not include CU patients with prior exposure or failure to previous biologics. Therefore, there is still limited data from clinical trials on the efficacy of ACT in CU patients who are refractory to biologic treatment. The available data on ACT in patients with Crohn’s disease is also limited, which poses a particular challenge in patients with complicated disease.
Combination therapy led to clinical remission in 54.5% of patients
The open-label phase 4 EXPLORER study investigated the efficacy of a triple combination therapy of vedolizumab, adalimumab and methotrexate in biologically untreated patients with Crohn’s disease (CD) at intermediate to high risk, as determined by an endoscopic score for CD (SES-CD) >7 (or >4 for isolated ileal disease). Eligible patients with Crohn’s disease were considered to be at medium to high risk of complications. An interim analysis of 55 patients treated with the triple therapy was performed (vedolizumab 300 mg i.v. on day 1 and weeks 2 and 6 and then every 8 weeks; adalimumab 160 mg s.c. on day 2, 80 mg at week 2 and then 40 mg every 2 weeks until week 26; methotrexate 15 mg orally weekly until week 34). After the triple and until week 34, each patient received vedolizumab monotherapy until week 102. At week 26, clinical response and endoscopic remission were observed in 54.4% and 34.5%, respectively. There were no safety signals associated with triple therapy. The current RCT evidence on ACT in the treatment of IBD is summarized in Table 1 .
The EXPLORER trial suggests the efficacy of a combination of vedolizumab, adalimumab and methotrexate in improving endoscopic response and remission in biologic-naïve CD patients at intermediate to high risk of complications. However, the lack of a controlled arm in the study prevents clear evidence of the therapeutic efficacy of ACT in CD, Dr. Wetwittayakhlang and Dr. Lakatos said.
Weak evidence from real-world studies
In terms of observational real-world data, a large multicenter study reported on 98 patients who started combination therapy for active IBD (67%), active immune-mediated inflammatory diseases (IMIDs) or extraintestinal manifestations (EIMs, 22%) or both (10%) after multiple biologics had failed in them. The disease activity of IBD was clinically improved in 70% of patients, and 81% of patients with IMIDs/EIMs. Another retrospective study reported on 92 patients who received combined biologic therapy for active IBD or EIMs. The most common combinations were vedolizumab and ustekinumab (32%) or vedolizumab and anti-TNF (31%). The clinical response rates at 3 and 6 months were 46% and 34% respectively. A retrospective study by Glassner et al. examined 50 patients with IBD who were treated with various combinations of biologics or small-molecule therapies. Approximately 50% received vedolizumab plus anti-IL-12 and IL-23 (ustekinumab) for persistent disease activity (n=47) or concurrent rheumatologic or dermatologic disease (n=3). After 4 months, significantly more patients were in clinical remission (50% vs. 14%, p=0.0018) and after 8 months in endoscopic remission (34% vs. 6%, p=0.0039) than at baseline.
Another case series showed that dual biologic therapy was safe and effective in 22 patients with severe refractory CD who had received a total of 24 dual biologic treatments after multiple failed biologic therapies. Clinical response and clinical remission were observed in 50% and 41% of patients, respectively. Endoscopic improvement and remission were observed in 43% and 26%, respectively. The presence of active perianal fistula decreased from 50% at baseline to 33% after treatment. Similarly, the efficacy of combining tofacitinib with other biologic therapies was reported in two retrospective cohort studies.
A meta-analysis by Alayo et al. evaluated the efficacy of ACT with different combination regimens. In patients receiving vedolizumab plus tofacitinib, the pooled clinical response and remission rates were 59.9% (95% CI 37.2-80.8) and 47.8% (95% CI 19.0-77.4), respectively. For vedolizumab plus ustekinumab, the pooled clinical response and remission rates were 83.9% (95% CI 66.4-96.8) and 47.0% (95% CI 14.5-80.7), respectively. Patients who received vedolizumab plus anti-TNF had pooled endoscopic/radiologic response and remission rates of 38.2% (95% CI 19.5-58.4) and 18.0% (95% CI 1.6-41.8), respectively. And in patients treated with tofacitinib plus vedolizumab, the corresponding rates were 46.2% (95% CI 20.4-73.0) and 24.6% (95% CI 6.4-47.6).
According to Dr. Wetwittayakhlang and Dr. Lakatos, observational studies from the real world, while informative, leave open whether ACT can raise the therapeutic ceiling in IBD in the complexities of a real-life clinical setting, as most patients receiving ACT have not responded or responded inadequately to biologic therapy. Therefore, the most reliable available data should be considered, mainly from a limited number of clinical trials involving patients with ACT who had not yet received biologic agents.
Promising therapeutic option for effective disease control
ACT has emerged as a potential new therapeutic strategy aimed at improving therapeutic efficacy and overcoming the plateau observed with currently available therapies. In highly selected patients with a high-risk disease phenotype (such as patients with extensive small bowel involvement in Crohn’s disease or patients who do not respond to multiple therapies), the use of ACT for early disease control may be useful to prevent disease progression and complications. During the induction phase, simultaneous co-induction with a combination of biologics or small molecules can be used to maximize disease control through synergistic effects. Once disease remission is achieved, monotherapy with one of the combined agents can be used to maintain disease remission.
Another aspect, according to the authors, that should be considered when applying the ACT strategy in clinical practice is the potential for changes in safety signals with prolonged exposure to multiple immunosuppressants. Although safety concerns have not been a major focus to date, it is important to recognize that the existing data come from small studies with limited follow-up periods. Therefore, further large prospective studies with longer follow-up periods are needed to fully understand the efficacy and safety of this strategy. Uncertainties also remain as to whether the combination should be used exclusively for the induction phase or extended to maintenance therapy.
Recent results such as those of the VEGA study show that the combination of novel biologics achieves superior therapeutic efficacy in biologically naïve patients with moderately to severely active UC, both clinically and endoscopically, compared to monobiologic therapy. However, the data is often limited. Looking ahead, ongoing studies such as the DUET-CD and DUET-UC trials (evaluating the combination of guselkumab and golimumab in refractory IBD patients) and the VICTRIVA trial (evaluating the combination of vedolizumab and upadacitinib) promise to provide additional data on the efficacy of ACT in Crohn’s disease and ulcerative colitis patients who have failed biologic therapy, Dr. Wetwittayakhlang concluded. Wetwittayakhlang and Dr. Lakatos.
Literature:
- Wetwittayakhlang P, Lakatos PL: Advanced combination therapy: is it the best way to break the therapeutic ceiling? Therapeutic Advances in Gastroenterology 2024; 17; doi: 10.1177/17562848241272995.
GASTROENTEROLOGY PRACTICE 2024; 2(2): 22-23