Some exciting studies were presented on renal cell carcinoma at this year’s ASCO annual meeting. Especially for localized tumors, clinical practice may change in the near future. For the first time, checkpoint inhibitors appear to be an effective option for adjuvant therapy. And there are also new findings in the first- and second-line treatment of advanced renal cell carcinoma.
Researchers have been looking into potential adjuvant therapies for renal cell carcinoma for years – so far without success. Unfortunately, no progress could be achieved by the use of tyrosine kinase inhibitors. Thus, a number of studies exist that showed no or minimal survival benefit of adjuvant treatment with sunitinib, sorafenib, pazopanib, and axitinib – with substantial toxicity [1–5]. Now, at the ASCO Annual Meeting, Keynote-564 was the first to present a study showing promise for adjuvant treatment after nephrectomy. This could soon change clinical practice for good. This is because currently only tumor follow-up is recommended after nephrectomy, regardless of tumor size and grading. However, it has been known for some time that these factors have a significant impact on prognosis [6]. Thus, it stands to reason that patients from the high-risk group in particular – for example, those with sarcomatoid dedifferentiated or large tumors – might benefit from adjuvant treatment after nephrectomy.
Adjuvant therapy: focus on checkpoint inhibitors
Whereas over the past decade the focus has been on tyrosine kinase inhibitors in the adjuvant setting, the focus is now on immunotherapy using checkpoint inhibitors. Currently, four studies are ongoing investigating the use of pembrolizumab, atezolizumab and nivolumab as monotherapy or in combination with ipilimumab (Tab. 1). Although the inclusion criteria for all studies differ somewhat, the focus is always on patients from the intermediate- and high-risk groups. A unique feature of both the Keynote 564 study and the IMmotion010 study is that sufferers after successful metastatic surgery within the first year after primary diagnosis could also be included.
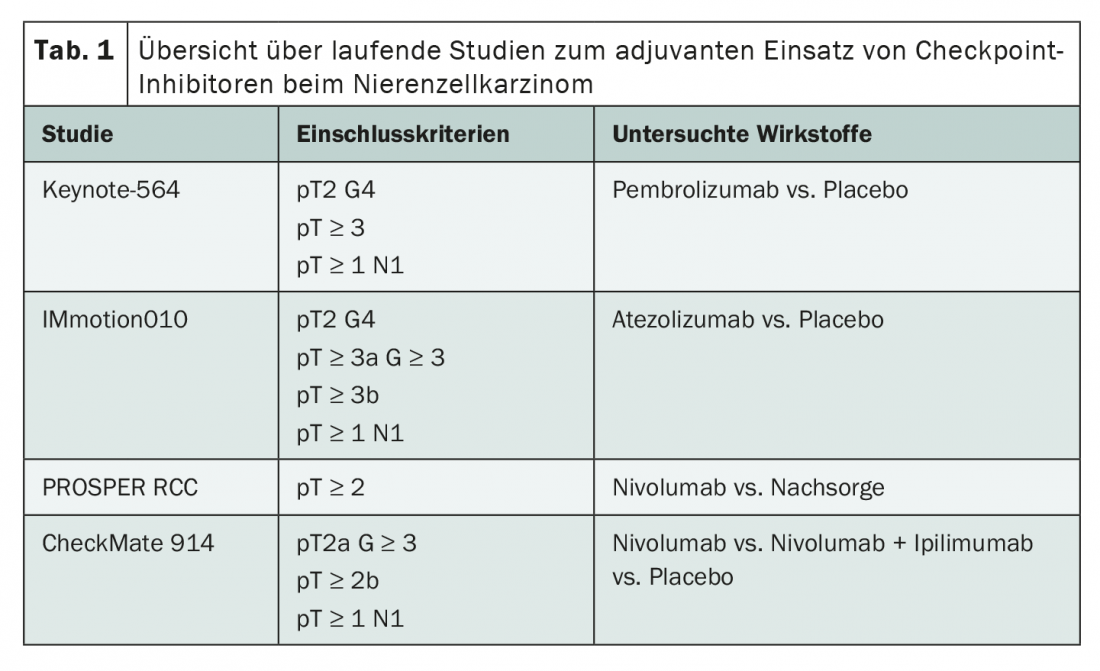
Initial results from Keynote-564 have now been presented at the ASCO Annual Meeting [7]. In the phase III trial, enrolled patients received either pembrolizumab or placebo for one year after nephrectomy. The median follow-up was 24.1 months. Both disease-free survival (DFS) and overall survival (OS) showed statistically significant benefits of adjuvant treatment with pembrolizumab – despite the still relatively short follow-up period. Thus, the DFS rate at 24 months was 77.3%, compared with 68.1% in the control arm (hazard ratio 0.68, p=0.001). The OS rate at 24 months was 96.6% in the intervention group and 93.5% under placebo (hazard ratio 0.54, p=0.0164). These effects were present across all subgroups and were similar in magnitude. Patients who had received prior successful metastasectomy appeared to benefit particularly from adjuvant immunotherapy. There were no surprises with regard to compatibility. As expected from the monotherapy studies, serious adverse events occurred in approximately 20% of patients, which corresponded to the known side effect spectrum of pembrolizumab. More mature data from the Keynote 564 trial, as well as preliminary findings from the other immunotherapy trials, remain to be seen to assess the true effects of treatment. However, after many unsuccessful years, an option for adjuvant therapy of renal cell carcinoma may now emerge after all, especially for high-risk patients.
Advanced renal cell carcinoma: first-line therapy in transition
Several findings were also presented at the ASCO Annual Meeting in the area of first-line therapy for advanced renal cell carcinoma. With the introduction of various combination therapies of immunotherapeutics and tyrosine kinase inhibitors, treatment is in a state of flux. Currently, different therapies are recommended depending on the risk profile (Table 2) [8]. Recently, the third-generation tyrosine kinase inhibitor (TKI) cabozantinib was also approved for first-line treatment in combination with nivolumab; this option is not yet covered in the guideline. A good data base also exists for treatment with lenvatinib + pembrolizumab, although approval is pending [9,10]. Currently, lenvatinib is approved in Switzerland only for second-line therapy after VEGF inhibitor [11].
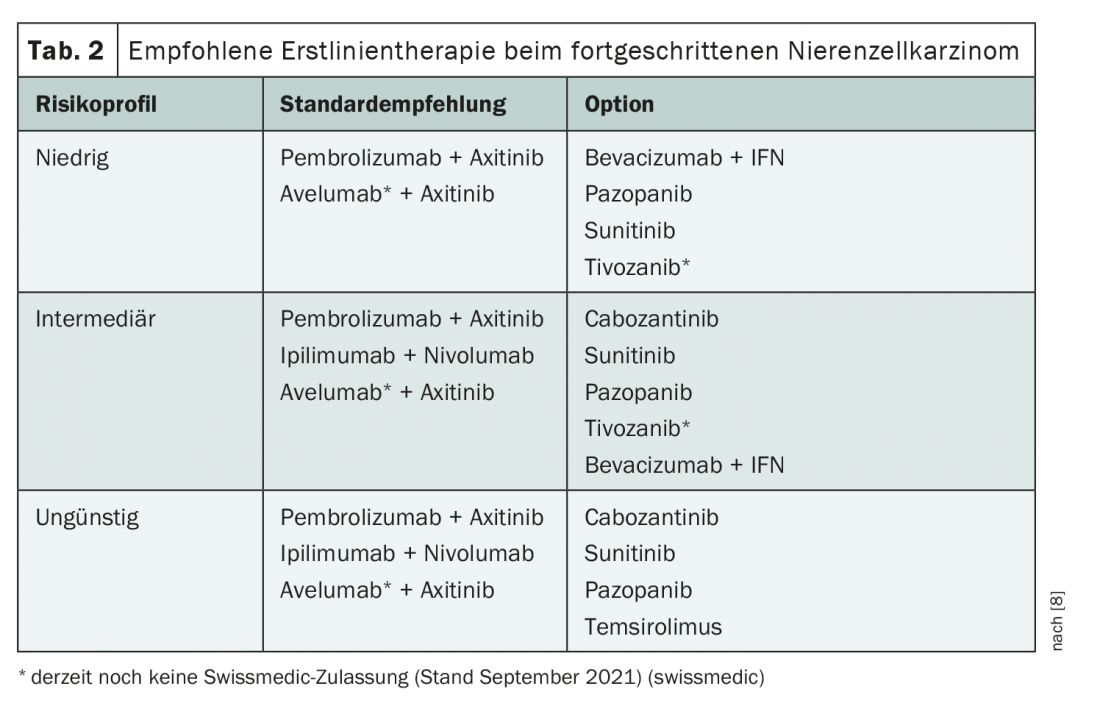
Among the different options, the current focus is on whether TKI-immunotherapeutic (IO) combinations can keep pace in the longer term with the IO-IO combination nivolumab/ipilimumab, for which a longer follow-up period already exists. To date, data for the combinations nivolumab/ipilimumab, axitinib/pembrolizumab, cabozantinib/nivolumab, and also for the not-yet-approved combination lenvatinib/pembrolizumab show similar results with respect to survival, with a hazard ratio of approximately 0.66 compared with monotherapy using sunitinib [9,12–16]. However, longer-term follow-up is lacking for IO-TKI combinations. One was presented for axitinib/pembrolizumab at this year’s ASCO annual meeting [15]. There were similar OS and PFS rates at the different time points as in the studies of the IO-IO combination nivolumab/ipilimumab. At 36 months, the PFS rate with both therapies was approximately 30% each [14,15]. This is certainly good news, but further data on the duration of response remain to be seen. The bottom line is that the follow-up of the TKI-IO combination axitinib/pembrolizumab shows consistent data to date with no new safety concerns.
In addition, data on the new TKI-IO combinations lanvatinib/pembrolizumab and cabozantinib/nivolumab were presented at the ASCO Annual Meeting. While these are clearly superior to sunitinib therapy alone in intermediate- and high-risk patients, patients with favorable risk profiles have so far shown significant PFS prolongation but without OS effect [10,17]. This could be due to the relatively short follow-up period, but indicates that the benefit of the dual strategy is potentially greater in the intermediate- and high-risk groups than in the low-risk population. Whether combinations with third-generation TKIs will replace other TKI-IO combinations in the future – for example, those with axitinib – currently remains to be seen.
News from the second line
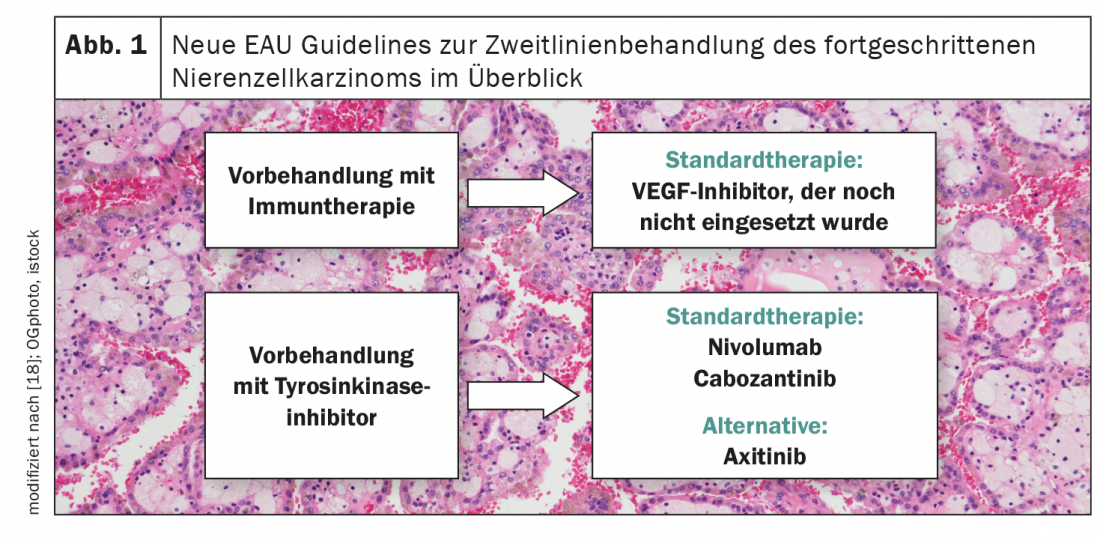
Solid recommendations remain lacking in renal cell carcinoma after failure of first-line therapy, especially if immunotherapeutics were used in the first line of treatment (Fig. 1) [18]. To date, three prospective studies exist in this setting – all single-arm. While a median PFS of 8.8 months was observed with axitinib treatment, this was 7.4 months with pazopanib therapy and 6.8 months with sunitinib administration [19-21]. The CANTATA study presented at the ASCO Annual Meeting now evaluated the efficacy of cabozantinib/telaglenastat in the second-line setting and compared this combination therapy to cabozantinib/placebo [22]. Although there was no benefit to adding telaglenastat, cabozantinib emerged as a promising candidate for second-line therapy of advanced renal cell carcinoma. Thus, the median PFS in the control arm was 9.3 months with an objective response rate of approximately 30%.
Congress: ASCO Annual Meeting
Literature:
- Ravaud A, et al: Adjuvant sunitinib in high-risk renal cell carcinoma after nephrectomy. N Engl J Med. 2016; 375(23): 2246-2254.
- Eisen TQG, et al: Primary Efficacy analysis results from the SORCE trial (RE05): Adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: an international, randomised double-blind phase III trial led by the MRC CTU at UCL. ESMO Congress 2019, Proffered Paper 2 – Genitourinary tumours, non-prostate, Abstract #2483.
- Motzer RJ, et al: Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J Clin Oncol. 2017; 35(35): 3916-3923.
- Haas NB, et al: Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016; 387(10032): 2008-2016.
- Gross-Goupil M, et al: Axitinib vs placebo in patients at high risk of recurrent renal cell carcinoma (RCC): ATLAS trial results. ESMO Congress 2018, Proffered paper session – Genitourinary tumours, non prostate, Abstract #1286.
- Gettman MT, et al: Pathologic staging of renal cell carcinoma: significance of tumor classification with the 1997 TNM staging system. Cancer. 2001; 91(2): 354-361.
- Choueiri TK, et al: Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for patients with renal cell carcinoma: Randomized, double-blind, phase III KEYNOTE-564 study. ASCO Annual Meeting 2021, Abstract #LBA5.
- AWMF: S3-Leitlinie Diagnostik, Therapie und Nachsorge des Nierenzellkarzinoms, Langversion 2.0, Stand August 2020.
- Motzer R, et al: Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021; 384(14): 1289-1300.
- Grünwald V, et al: Analysis of the CLEAR study in patients (pts) with advanced renal cell carcinoma (RCC): Depth of response and efficacy for selected subgroups in the lenvatinib (LEN) + pembrolizumab (PEMBRO) and sunitinib (SUN) treatment arms. ASCO Annual Meeting 2021, Abstract #4560.
- www.swissmedicinfo.ch (last accessed 09/15/2021)
- Albiges L, et al: Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open. 2020; 5(6): e001079.
- Choueiri TK, et al: Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Annals of Oncology. 2020; 31: S1142-1215.
- Motzer RJ, et al: Survival outcomes and independent response assessment with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma: 42-month follow-up of a randomized phase 3 clinical trial. J Immunother Cancer. 2020; 8(2).
- Rini BI, et al: Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma (ccRCC): Results from 42-month follow-up of KEYNOTE-426. ASCO Annual Meeting 2021, Abstract #4500.
- Plimack ER, et al: Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced renal cell carcinoma (RCC): Updated analysis of KEYNOTE-426. Journal of Clinical Oncology. 2020; 38(15_suppl).
- Apolo AB, et al: Nivolumab plus cabozantinib (N+C) versus sunitinib (S) for advanced renal cell carcinoma (aRCC): outcomes by baseline disease characteristics in the phase 3 CheckMate 9ER trial. ASCO Annual Meeting 2021, Abstract #4553.
- Ljungberg B, et al: EAU Guidelines: Renal Cell Carcinoma 2021. https://uroweb.org/guideline/renal-cell-carcinoma/ (last accessed 09/15/2021).
- Ornstein MC, et al: Individualised axitinib regimen for patients with metastatic renal cell carcinoma after treatment with checkpoint inhibitors: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2019; 20(10): 1386-1394.
- Powles TB, et al: A phase II study of patients with advanced or metastatic renal cell carcinoma (mRCC) receiving pazopanib after previous checkpoint inhibitor treatment. Annals of Oncology. 2020; 31: S564.
- Grande E, et al: INMUNOSUN-SOGUG trial: A prospective phase II study to assess the efficacy and safety of sunitinib as second-line (2L) treatment in patients (pts) with metastatic renal cell cancer (RCC) who received immunotherapy-based combination upfront. Journal of Clinical Oncology. 2020; 38(15_suppl).
- Tannir NM, et al: CANTATA: Primary analysis of a global, randomized, placebo (Pbo)-controlled, double-blind trial of telaglenastat (CB-839) + cabozantinib versus Pbo + cabozantinib in advanced/metastatic renal cell carcinoma (mRCC) patients (pts) who progressed on immune checkpoint inhibitor (ICI) or anti-angiogenic therapies. ASCO Annual Meeting 2021, Abstract #4501.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(4): 33-34.