In treatment-resistant severe cases, a biologic representative of a new substance class, vedolizumab, is now available in addition to conventional standard medication and anti-TNFα. Several evaluations of different therapies from a clinical and health economic point of view came up with exciting results. The VARSITY study was the first head-to-head comparison of first-line biologics.
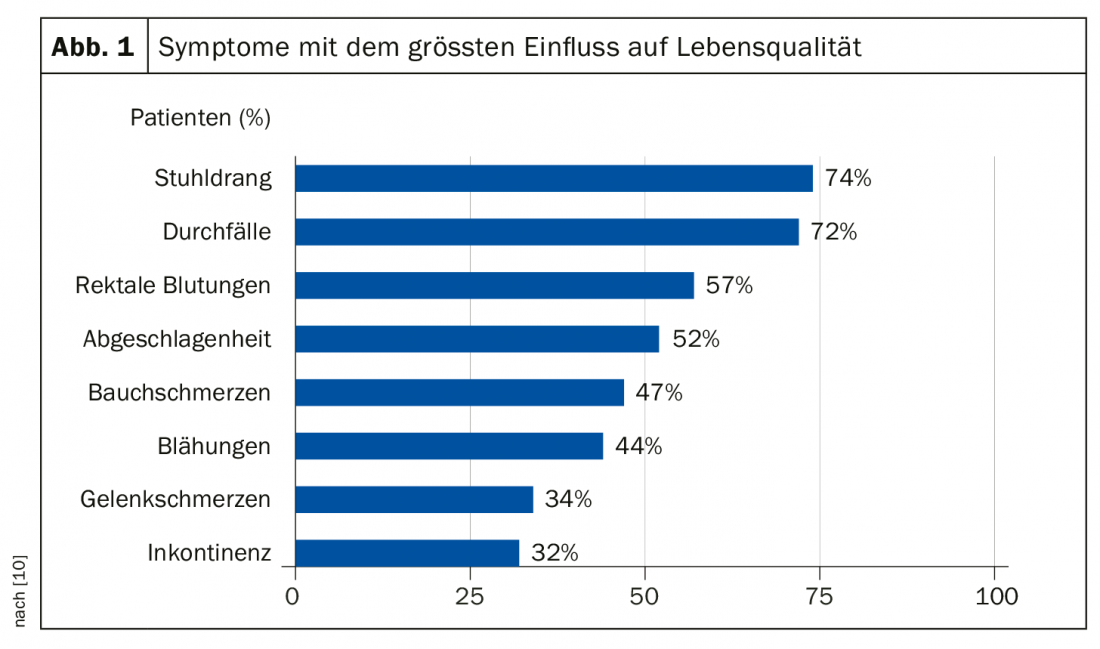
Ulcerative colitis and Crohn’s disease are two forms of chronic inflammatory bowel disease (IBD), referred to as Irritable Bowel Syndrome (IBS). The disease can occur at any age, the majority of the age group affected is 20-40 years. Symptoms vary interindividually and range from mild abdominal pain to colicky symptoms and numerous bouts of diarrhea. Figure 1 lists the symptoms that have the greatest impact on quality of life according to an exploratory study (UC-LIFE survey, n=436).
The course of IBD is typically relapsing; phases of low disease activity may be followed by severe relapses. To control symptoms and improve quality of life, patients often require lifelong treatment. The etiology of IBD is not yet fully understood; it is assumed that there is a multifactorial structure and a genetic disposition. Chronic inflammation in the intestine is due to a dysregulation of the immune system.
Therapeutically, the focus is on containing the inflammation to relieve symptoms and reduce relapses. Clinical remission is the primary goal of treatment, and additional mucosal healing (“deep remission”) to histologic healing (“intestinal healing”) may be achieved [1]. Mucosa healing has been shown to be a prognostically favorable factor [2].
Conventional therapy put to the test
The COCOS study (“Course and Costs of Conventional Therapies in Patients with Inflammatory Bowel Disease”) examined treatment and costs of patients not treated with biologics [3,7]. The focus was an analysis of the care situation of biologics-naïve patients with Crohn’s disease and ulcerative colitis using real-world data and identification of patients with signs of disease activity and need for therapy escalation.
The sample included data from patients of statutory health insurance with Crohn’s disease and ulcerative colitis of at least moderate severity (≥1 prescription of a systemic corticosteroid or oral budesonide or immunosuppressant and no biologics prescription two years before or one year after the index date, respectively). The follow-up period was at least 12 months. It was shown that more than one-third of IBD patients without biologics had disease activity, according to PD Dr. med. Bernd Bokemeyer, Minden, Germany (D). Disease activity was defined as the presence of at least one of the following conditions during the follow-up period: (a) ≥2 prescriptions of systemic corticosteroids, (b) ≥2 prescriptions of oral budesonide (sensitivity for both: ≥3); (c) ≥1 CED-related surgery; (d) ≥7 days of CED-related hospitalizations [3].
Furthermore, the analysis showed that a large proportion of patients receive only steroids, and patients managed by specialists are more frequently treated with immunosuppressants. Of the 9471 IBD patients not treated with biologics, more than 44% could be identified with a need for therapy escalation at 12 months follow-up [3]. Patients in need of therapy escalation were more likely to have therapy with steroids during the course. Thus, among patients requiring therapy escalation, 69.9% received only corticosteroids during follow-up and only 7.1% received a conventional immunosuppressant. Patients treated with corticosteroids alone had fewer visits with a specialist compared with patients receiving an immunosuppressant. Patients with a need for therapy escalation resulted in higher costs compared to patients without a need for escalation. Another conclusion of the COCOS study was that gastroenterologists should be more involved in patient care for optimal treatment quality.
First head-to-head comparison of first-line biologics
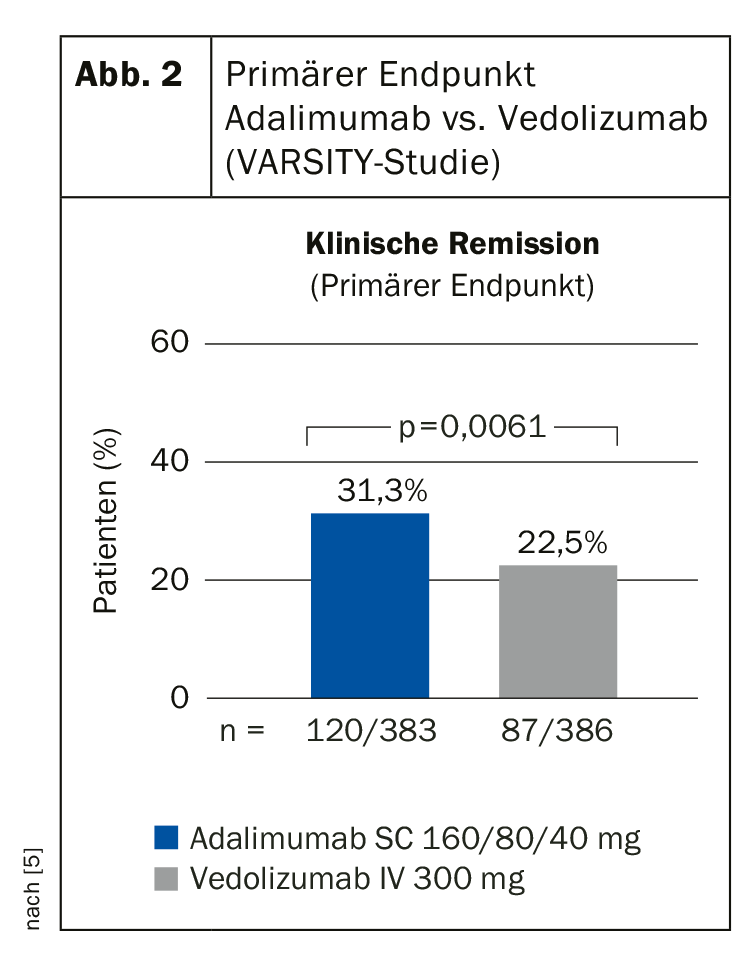
PD Dr. Irina Blumenstein, Klinikum der Johann-Wolfgang Universität, Frankfurt am Main, Germany, presented data from the VARSITY trials [4]. VARSITY is the first head-to-head comparison of vedolizumab and adalimumab in patients with moderate-to-severe active ulcerative colitis. The double-blind, active-controlled phase IIIb trial in double-dummy design involved 330 study centers in 37 countries [5]. Patients (n=769) were randomized 1:1 to receive the gut-selective integrin antagonist vedolizumab i.v. (Entyvio®, Takeda) plus placebo s.c. or the TNFα antagonist adalimumab s.c. plus placebo i.v. Another inclusion criterion was lack of response to conventional therapy. 25% had been pretreated with a TNFα antagonist.
The results of VARSITY support therapy with vedolizumab as a first-line biologic. Furthermore, all approved biologics were shown to be effective compared to placebo. In biologics-naive patients, infliximab and vedolizumab performed best in terms of clinical remission and mucosa healing. Vedolizumab showed the lowest rate of serious adverse events and infections. Clinical remission as the primary endpoint was defined as ≤2 points in the total Mayo score or none of the subscores >1 point at week 52. Significantly more patients achieved this endpoint in the vedolizumab condition compared with the adalimumab condition (31.3% vs. 22.5%) (Fig. 2). There was also significant superiority in the secondary endpoint (mucosa healing, defined as ≤1 point in the endoscopic Mayo subscore at week 52) with vedolizumab (39.7% vs. 27.7%).
The efficacy and safety profile of vedolizumab from previous studies was confirmed. An adverse event was observed in a total of 62.7% of patients and in 69.2% in the adalimumab group. The exposure-adjusted infection rate was 33.5% with vedolizumab vs. 43.5% with adalimumab.
The findings of the VARSITY study confirm the results of a meta-analysis on the efficacy of vedolizumab in daily practice in IBD patients [6]. At treatment week 14, 32% of ulcerative colitis patients and 30% of Crohn’s disease patients were in remission, compared with 46% and 30%, respectively, at month 12 after baseline. 30%. Rates of corticosteroid-free remission were 26% in ulcerative colitis patients at week 14 and 42% at one year. In Crohn’s disease, these rates were 25% and 31%, respectively. A serious adverse event occurred in 9%. The authors concluded that these data support the long-term benefit-risk profile of vedolizumab [6].
Therapy algorithm at a glance
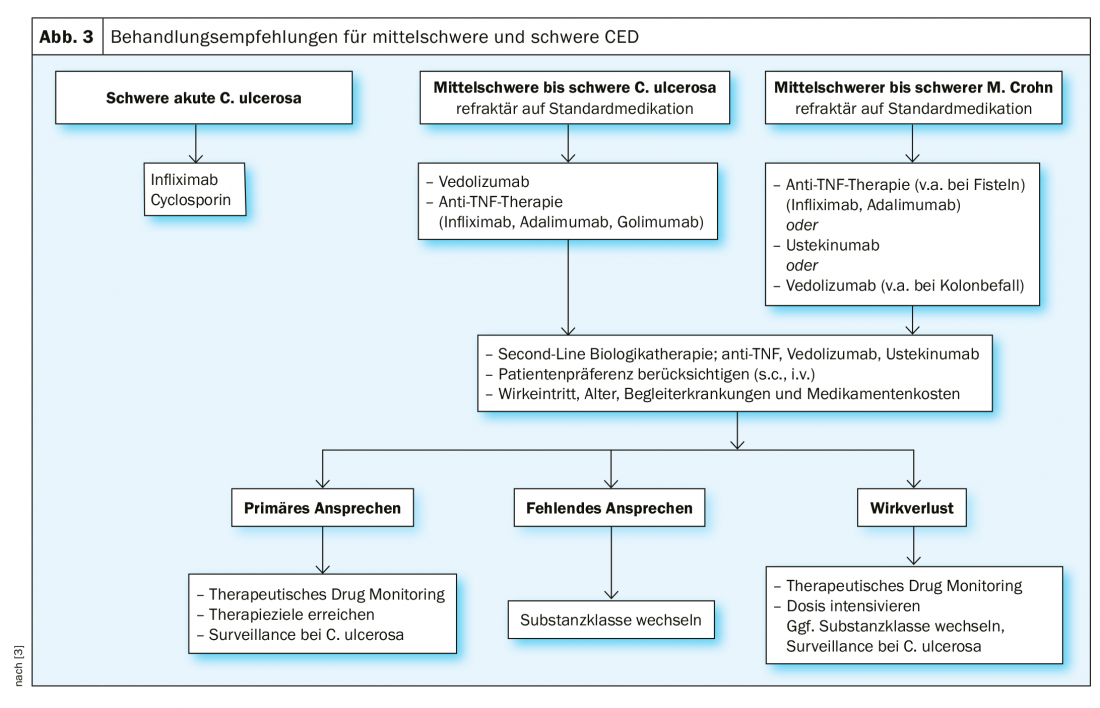
The treatment of colitis depends on the severity of the symptoms and the sites of inflammation in the intestine. In mild ulcerative colitis, 5-aminosalicylic acid preparations are often used initially (e.g., mesalazine or sulfasalazine), and possibly also corticosteroids. The focus here is on reducing inflammation. After the acute inflammation has subsided, 5-ASA preparations are usually prescribed as maintenance therapy to reduce the risk of a new flare-up. In patients with a form of ulcerative colitis characterized by frequent relapses or in the case of very active disease, immunosuppressants such as azathioprine or 6-mercaptopurine may also be prescribed [8]. If a very active and aggressive episode of ulcerative colitis is present, intravenous corticosteroids may need to be used. If this does not provide relief, other immunosuppressants such as cyclosporine may be tried. In a severe form of ulcerative colitis, biologics such as infliximab, adalimumab, golimumab, and vedolizumab are also used today.
In Crohn’s disease, the treatment depends on how severely the bowel is affected and which part of the bowel is affected. In the presence of active inflammatory foci, corticosteroids are usually used to relieve acute symptoms. For mild inflammation, 5-aminosalicylic acid preparations such as mesalazine or sulfasalazine may also be prescribed [8]. The use of immunosuppressants such as azathioprine, 6-mercaptopurine, or methotrexate is recommended only for more aggressive forms of Crohn’s disease. If there is a lack of response to conventional drug therapy, biologics such as adalimumab, certolizumab, infliximab, or vedolizumab are available.
Vedolizumab for treatment resistance
The class of anti-TNFα inhibitors has revolutionized the treatment of IBD. The efficacy and safety of adalimumab in moderate to severe active ulcerative colitis was demonstrated in the ULTRA 1 and 2 studies. There are positive long-term data for golimumab for this indication from the PURSUIT extension study [12]. For Crohn’s disease, of the representatives of the TNFα antagonists, adalimumab and infliximab are available.
However, there are patients with lack of response, decreasing efficacy, or intolerance regarding TNFα antibodies. For these, the anti-integrin antibody vedolizumab is an alternative [9]. This agent, which performed well in the HEAD-to-HEAD study VARSITY, has been approved in Switzerland since 2015 for moderate to severe active ulcerative colitis and Crohn’s disease in cases of inadequate response or intolerance to conventional therapy or TNFα inhibitor [8]. It has a gut-selective effect and binds to the α4β7-integrin of other blood cells, preventing them from triggering an inflammatory response in the intestinal tissue.
Source: DGIM Wiesbaden (D)
Literature:
- Hirschmann K, Hoffmann JC: Drug Therapy 2018; 36: 286-292. www.aerztekammer-bw.de/10aerzte/20fortbildung/20praxis/88arzneimitteltherapie/1809.pdf
- Walsh A, Palmer R, Travis S: Mucosa healing as a target of therapy for colonic inflammatory bowel disease and methods to score disease activity. Gastrointest Endosc Clin J Am 2014; 24: 367-378.
- Bokemeyer B: Care and therapy escalation in IBD with biologics: Who? When? With what? How?, slide presentation PD Dr. med. Bernd Bokemeyer, Minden (D), Industry Symposium, DGIM May 6, 2019, Wiesbaden.
- Blumenstein I: Care and therapy escalation in IBD with biologics: Who? When? With what? How?, slide presentation PD Dr. Irina Blumenstein, Klinikum der Johann-Wolfgang Universität, Frankfurt am Main, DGIM May 6, 2019, Wiesbaden.
- Schreiber S, et al: J Crohns Colitis 2019; 13 (Suppl 1): S612-613 (Abstract OP34).
- Schreiber S, et al: Systematic review with meta-analysis: real-world effectiveness and safety of vedolizumab in patients with inflammatory bowel disease. J Gastroenterol 2018; 53 (9): 1048-1064.
- Bokemeyer B: Analysis of real-world data on disease activity of patients with inflammatory bowel disease without biologics therapy – COCOS study. Z Gastroenterol 2019; 57(09): e204. DOI: 10.1055/s-0039-1695155.
- IBDnet: Swiss Research and Communication Network on Inflammatory Bowel Disease, www.ibdnet.ch
- Scribano ML: Vedolizumab for inflammatory bowel disease: from randomized controlled trials to real-life evidence. World J Gastroenterol. 2018; 24(23): 2457-2467.
- Carpio D: Perception of disease burden and treatment satisfaction in patients with ulcerative colitis from outpatient clinics in Spain: UC-LIFE survey. Eur J Gastroenterol Hepatol 2016; 28: 1056-1064.
- Hanauer S, et al: Rapid Changes in Laboratory Parameters and Early Response to Adalimumab: A Pooled Analysis From Patients With Ulcerative Colitis in Two Clinical Trials. Journal of Crohns and Colitis, 13(9): 1227-1233.
- Reinisch W: Long-term Benefit of Golimumab for Patients with Moderately-to-Severely Active Ulcerative Colitis: Results from the PURSUIT-Maintenance Extension. J Crohns Colitis 2018. doi: 10.1093/ecco-jcc/jjy079. [Epub ahead of print].
HAUSARZT PRAXIS 2019; 14(10): 30-32 (published 10/24/19, ahead of print).