For both germ cell and epithelial ovarian tumors, the first line of therapy is surgery and chemotherapy. However, there has been a lot of progress over the last few years, especially in ovarian cancer. Thus, there is a growing data base on maintenance therapy using bevacizumab and/or PARP inhibitors.
While targeted oncologic approaches have not yet gained acceptance in germ cell tumors of the ovary, they are now established in everyday practice with PARP inhibitors in the maintenance therapy of epithelial ovarian tumors and have been approved by regulatory authorities. Nevertheless, some questions remain unanswered, such as the ideal combination and sequence of active ingredients. Direct comparisons of the various substances with each other are also lacking. And it’s not just the newly introduced agents that are unclear, but also the standard first-line therapies for ovarian tumors. For example, should surgery be postponed to a later date to increase the chance of R0 resection? And how might we succeed in improving the care of patients with germ cell tumors in view of the low case numbers?
Germ cell tumors: learning from men?
Although germ cell tumors of the ovary have a good prognosis after successful surgery, studies on optimal therapy still need to be improved. For example, few data exist on the value of radiotherapy, and it is not certain that chemotherapy is actually necessary in all cases to the extent that it is given today. Even in the recurrent setting, the study landscape is sparse – despite extremely poor prognosis and thus highmedical need . While exciting findings on the treatment of seminoma and non-seminoma are repeatedly published in men, these are rare in female germ cell tumors. One strategy, therefore, takes the approach of transferring study results from male or mixed cohorts to female patients. This is because germ cell tumors are much more common in men, with an incidence of 6/100,000 per year, than in women, with an incidence of 0.2/100,000/year. On the one hand, this approach is an opportunity, but it also harbors dangers, warned Prof. Viola Heinzelmann, Head of the Gynecological Tumor Center and Chief of Gynecology/Gynecological Oncology at the University Hospital Basel, at this year’s congress of the German, Austrian and Swiss Societies of Hematology and Medical Oncology in Berlin. In many respects – such as available biomarkers, age of onset, and treatment strategies – male and female germ cell tumors are indeed similar, he said. Nevertheless, he said, there are relevant differences such as increasing incidence in the male sex and staging.
Currently, first-line therapy – largely extrapolated from study data of male germ cell tumors – consists particularly of surgery (laparotomy or minimally invasive) and chemotherapy. Rupture should be avoided to prevent spread of the tumor. Fertility-preserving surgery can be performed in most cases, but hysterectomy and adnexectomy are still recommended after family planning has been completed [1]. In patients suffering from dysgerminoma or immature stage IA teratoma, postoperative observation is sufficient, whereas all other patients should receive chemotherapy within ten days after surgery [1]. This has lasting effects on fertility and may be unnecessary in stage IA for all germ cell tumors – but reliable data are lacking here.
Recurrences should also be surgically removed whenever possible. In particular, vinblastine/ifosfamide/cisplatin (VeIP) or paclitaxel/ifosfamide/cisplatin are used for second-line treatment. However, curative carboplatin-based approaches exist with good results [2]. Other experimental options include high-dose chemotherapy with autologous stem cell transplantation, accelerated or standard BEP (bleomycin/etoposide/platinum), carboplatin/paclitaxel, and active surveillance [3]. There is a phase II study published in 2021 on the use of immunotherapy using checkpoint inhibitors, in which no benefit was observed with pembrolizumab administration [4]. Due to the high genomic instability, targeted therapies for germ cell tumors are rather not an option, according to Prof. Heinzelmann. More promising, he said, is an ongoing multicenter randomized trial comparing paclitaxel/carboplatin with bleomycin/etoposide/cisplatin in the relapsed setting. The results of this study are expected in 2024.
At this year’s congress of the European Society for Medical Oncology (ESMO), two interesting innovations were presented – at least in male germ cell tumors. Thus, in stage IIA/B seminoma, intensity reduction of therapy to a single course of carboplatin and radiotherapy appears to be warranted [5]. Also, cabazitaxel could provide a survival benefit in advanced germ cell tumors after two to three previous lines of treatment [6]. Whether these study results are also applicable to female patients remains to be investigated. Radiation is not part of the standard therapy in women, partly for reasons of fertility preservation and partly because of anatomical conditions. Randomized trials have not yet investigated this option in females. To address the insufficient data base for the treatment of female germ cell tumors, the two networks ENGOT (European Network of Gynaecological Oncological Trial groups) and GCIS (International Gynecological Cancer Intergroup Ovarian Cancer). which are to coordinate and initiate important studies in order to finally provide clarity in the case of dysgerminoma, teratoma and the like in women.
Focus on first-line therapy of ovarian cancer
In addition to germ cell tumors, the annual meeting of the DGHO, OeGHO, SSMO and SGH/SSH also addressed the much more common ovarian cancer. In particular, the focus was on whether neoadjuvant chemotherapy should be given before surgery. And maintenance therapy using bevacizumab and/or PARP inhibitor was also discussed. In addition to surgery and adjuvant chemotherapy, this is already part of the standard of care in the first line from stage III onwards [7]. While corresponding benefits in terms of progression-free survival (PFS) have already been demonstrated in the various pivotal studies, no published data on overall survival under the newly recommended maintenance therapy exist yet.
In principle, macroscopically complete resection is considered extremely important prognostically [8]. One is achieved in about 60% of cases, with a higher rate of R0 resection after neoadjuvant chemotherapy. Therefore, it is an obvious consideration to perform surgery only after primary chemotherapy has been completed. However, this approach does not seem to improve outcome [9,10]. Thus, according to current data, tumor freedom after primary surgery is superior to that after interval surgery. This is also reflected in the guidelines, which do not specify neoadjuvant chemotherapy [7]. However, the treatment sequence has only been studied in advanced, often primarily inoperable tumors. Thus, the question of neoadjuvant chemotherapy currently remains unanswered for those patients who are judged to be primarily operable. For this reason, the TRUST (Trial on Radical Upfront Surgical Therapy) trialwas initiated, which includes stage IIIB, IIIC, and IV patients who are judged to be operable without tumor. The evaluation of the data is in full swing and should finally clarify whether interval surgery might be superior to the standard of care after all in certain cases. One factor that should not be neglected here is surgery-associated morbidity and mortality. Patients with a high surgical risk should be better identified, according to Prof. Barbara Schmalfeldt, head of the Gynecological Cancer Center at the University Medical Center Hamburg-Eppendorf. These patients, approximately 10%, would benefit more from primary chemotherapy followed by reevaluation of surgery [11–13]. However, identifying such fragile patients is challenging. As an approach, the so-called “4 A” method was presented at the congress, whereby age, albumin, stage of disease (advanced disease) and general condition are taken into account in the treatment decision.
Despite convincing data for first-line maintenance therapy, surgery and, if possible, complete resection remain of great importance – this was also emphasized by Prof. Schmalfeldt. Thus, even with maintenance therapy, PFS was strongly dependent on tumor remnant [14].
The role of PARP inhibitors in high-grade serous ovarian cancer.
The most groundbreaking innovations in the treatment of ovarian cancer in recent years involve first-line maintenance therapy. After the introduction of the VEGF inhibitor bevacizumab in this indication, the PARP inhibitors niraparib and olaparib were also added in the presence of a BRCA mutation or other homologous recombination deficiency (HRD). A prerequisite for maintenance therapy with bevacizumab and/or PARP inhibitor is a response to first-line chemotherapy [15]. The benefit of VEGF inhibition – in which bevacizumab administration is started simultaneously with chemotherapy and continued after the end of chemotherapy – was confirmed at this year’s ASCO Annual Meeting. In contrast, extending treatment from 15 to 30 months provided no additional benefit [16]. The use of the PARP inhibitors olaparib and niraparib in first-line maintenance therapy also results in a proven PFS benefit over placebo as well as monotherapy using bevacizumab in HRD-positive tumors [17]. This has already led to Swissmedic approvals and corresponding amendments to the guidelines [7,15].
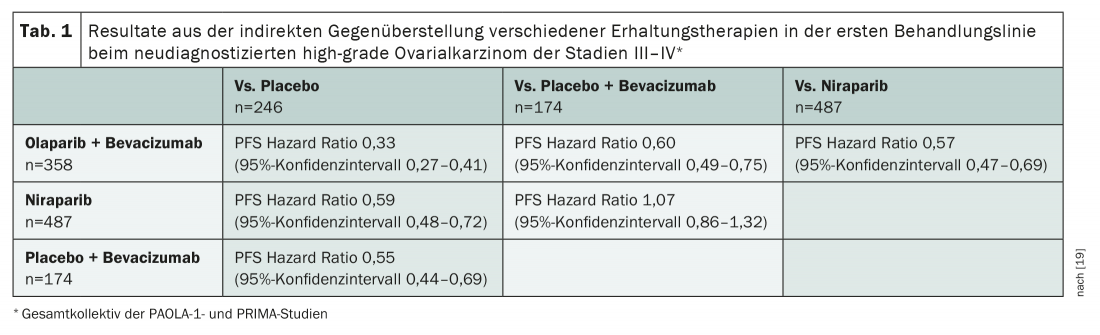
Although the benefit of PARP inhibitors in HRD-positive high-grade ovarian cancer is now hardly controversial, there are still some questions to be answered about their use. Thus, to date, there are no direct comparisons between the individual active ingredients. Also, combination therapy with bevacizumab continues to be controversial. Two population-adjusted indirect comparisons exist for this purpose based on the pivotal studies [18,19]. These are methodologically questionable – and extremely interesting. On the one hand, they provide evidence that simultaneous administration of a PARP inhibitor with bevacizumab confers advantages over PARP inhibitor monotherapy in terms of PFS. This option also seems to perform better compared to bevacizumab alone (Tab. 1) . If there is no contraindication for bevacizumab, the substance should therefore be used – in HRD-positive cases supplemented by a PARP inhibitor. If there are contraindications to bevacizumab, maintenance therapy using PARP inhibition alone can be started after chemotherapy in HRD-positive patients. PARP inhibitors – in this case olaparib, niraparib, and rucaparib – are also approved for recurrent platinum-sensitive ovarian cancer, regardless of mutation status.
With the increased use of targeted agents, the question of the optimal testing strategy is increasingly arising. One thing is clear: genetic testing should be offered in all cases [7]. While at some centers first tumor and then germline diagnostics are performed, elsewhere the opposite approach is preferred. An established standard is missing.
Source: Scientific Symposium “Update Ovarian Tumors” chaired by Sigrun Greil-Ressler and Hans Tesch, Oct. 04, 2021, Annual Meeting of the German, Austrian and Swiss Societies of Hematology and Medical Oncology, Berlin (D).
Literature:
- Brown J, et al: Gynecologic Cancer Intergroup (GCIG) consensus review for ovarian germ cell tumors. Int J Gynecol Cancer. 2014; 24(9 Suppl 3): S48-54.
- De Giorgi U, et al: Salvage high-dose chemotherapy in female patients with relapsed/refractory germ-cell tumors: a retrospective analysis of the European Group for Blood and Marrow Transplantation (EBMT). Ann Oncol. 2017; 28(8): 1910-1916.
- Uccello M, et al: Systemic anti-cancer treatment in malignant ovarian germ cell tumors (MOGCTs): current management and promising approaches. Ann Transl Med. 2020; 8(24): 1713.
- Tsimberidou AM, et al: Pembrolizumab in Patients with Advanced Metastatic Germ Cell Tumors. Oncologist. 2021; 26(7): 558-e1098.
- Papachristofilou A, et al: Single-dose carboplatin followed by involved-node radiotherapy as curative treatment for seminoma stage IIA/B: Efficacy results from the international multicenter phase II trial SAKK 01/10. ESMO Congress 2021, Proffered Paper Session – Genitourinary tumours, non-prostate 2, Abstract #LBA30.
- Baciarello G, et al: A prospective phase II trial of cabazitaxel in male patients with chemotherapy pre-treated metastatic germ-cell tumors: The CABA-GCT study. ESMO Congress 2021, Mini oral session – Genitourinary tumours, non-prostate, Abstract #655MO.
- AWMF S3-Leitlinie: Diagnostik, Therapie und Nachsorge maligner Ovarialtumoren, Version 5.0, September 2021.
- du Bois A, et al: Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009; 115(6): 1234-1244.
- Vergote I, et al: Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010; 363(10): 943-953.
- Kehoe S, et al: Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015; 386(9990): 249-257.
- Vergote I, et al: Neoadjuvant chemotherapy in advanced ovarian cancer: On what do we agree and disagree? Gynecol Oncol. 2013; 128(1): 6-11.
- Aletti GD, et al: Multidisciplinary approach in the management of advanced ovarian cancer patients: A personalized approach. Results from a specialized ovarian cancer unit. Gynecol Oncol. 2017; 144(3): 468-473.
- Ataseven B, et al: Pre-operative serum albumin is associated with post-operative complication rate and overall survival in patients with epithelial ovarian cancer undergoing cytoreductive surgery. Gynecol Oncol. 2015; 138(3): 560-565.
- Harter P, et al: Efficacy of maintenance olaparib plus bevacizumab by biomarker status in clinical higher- and lower-risk patients with newly diagnosed, advanced ovarian cancer in the PAOLA-1 trial. International Journal of Gynecologic Cancer. 2020; 30: A13-A4.
- Swissmedic drug information: www.swissmedicinfo.ch (last accessed 10/28/2021).
- Pfisterer J, et al: Optimal treatment duration of bevacizumab (BEV) combined with carboplatin and paclitaxel in patients (pts) with primary epithelial ovarian (EOC), fallopian tube (FTC) or peritoneal cancer (PPC): A multicenter open-label randomized 2-arm phase 3 ENGOT/GCIG trial of the AGO Study Group, GINECO, and NSGO (AGO-OVAR 17/BOOST, GINECO OV118, ENGOT Ov-15, NCT01462890). ASCO Annual Meeting 2021, Oral Abstract Session Gynecologic Cancer, Abstract #5501.
- Ray-Coquard I, et al: Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med. 2019; 381(25): 2416-2428.
- Vergote I, et al: Population adjusted indirect comparison of the SOLO1 and PAOLA-1/ENGOT-ov25 studies of olaparib with or without bevacizumab, bev alone and placebo in the maintenance treatment of women with newly diagnosed stage III/IV ovarian cancer with BRCA mutation. SGO Annual Meeting 2020, Abstract #35.
- Hettle R, et al: Population-adjusted indirect treatment comparison (PAITC) of maintenance PARP inhibitor (PARPi) with or without bevacizumab versus bevacizumab in women with newly diagnosed ovarian cancer (OC). ASCO Annual Meeting 2020, Poster Session Gynecologic Cancer, Abstract #6052.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(6): 19-22 (published 7/12/21, ahead of print).