The gold standard for acute stroke evaluation is native CT to exclude hemorrhage, after which iv thrombolytic therapy can be administered in the 4.5-hour time window currently allowed and after exclusion of possible contraindications. The greatest challenge in interdisciplinary stroke management is time-optimized patient selection for available therapies (iv thrombolytic therapy, ia recanalization, ia lysis, no therapy). Promising state-of-the-art imaging protocols aid in decision making in the acute setting and can assess penumbra and individual vascular collateral status within minutes. This may justify endovascular therapies outside the 4.5-hour time window in the presence of good collaterals or in the vertebrobasilar stromal area.
Acute stroke is the most common neurological emergency. The most frequent cause is acute occlusion of arteries supplying the brain. Restoring cerebral blood flow as quickly as possible is critical for successful treatment. The extent of tissue damage depends largely on ischemia time and available collaterals. The entire treatment chain must therefore be time-optimized: from the onset of the stroke, transport to the hospital, diagnostics, acute treatment, to the stroke unit. Follow-up and rehabilitation are equally important, but less time-critical.
The role of neuroradiology is in primary imaging diagnosis and neurointerventional revascularization. At the outset, the distinction between ischemic cerebral infarction (about 80%) and cerebral hemorrhage (the most common differential diagnosis) is clinically neurologically impossible. Only patients with ischemic stroke qualify for any form of revascularizing therapy. The gold standard for the evaluation of acute stroke is native computed tomography (native CT) because of its wide availability and high sensitivity to rule out cerebral hemorrhage. This is sufficient to initiate timely iv thrombolytic therapy if functionally relevant deficits and no contraindications (e.g., traumatic brain injury, recent surgery, derailed coagulation) are present. In 2010, according to new scientific evidence based on relevant results, the time window between symptom onset and therapy initiation for thrombolytic therapy was extended from 3 to 4.5 hours (ECASS III) [1].
Native CT is known to have low sensitivity for detecting cerebral infarction in the first few hours after infarct onset and varies with time interval and radiologic experience. Modern examination protocols (CT perfusion) significantly increase sensitivity [2], provide useful additional information on individual stroke and vascular collateral status, and help to make individualized treatment decisions. In addition to evidence-based iv thrombolytic therapy in a 4.5-hour time window, endovascular therapy approaches of interventional neuroradiology exist for proximal vessel occlusions, for example. These mechanical recanalization procedures were able to achieve better recanalization rates of occluded proximal cerebral arteries compared with iv thrombolysis [3], but did not significantly improve the functional outcome of treated patients in the larger, much-discussed, and new studies (IMS III) [4]. Here, further studies with optimized design, better patient selection, and uniform revascularization equipment remain to be performed. ia revascularization is currently used up to 6-8 hours after symptom onset, with no time limit for vertebrobasilar occlusions. The real challenge in neuroradiological assessment and interdisciplinary treatment of acute stroke lies in the decision for the individually best/effective therapy. The central question in the interdisciplinary stroke center is: Which patient benefits better from iv thrombolysis, ia recanalization, ia thrombolysis, or no therapy and when? In addition to age, infarct size, severity of neurological deficits, location and size of vessel occlusion, and time window, other factors (comorbidities, medication, individual vascular anatomy, and any contraindications) play an important role in the decision for or against a specific form of therapy. Helpful for decision making are newer CT techniques (CT perfusion), which together with native CT and CT angiography (CTA) in the acute setting rule out cerebral hemorrhage in a few minutes and allow useful conclusions about the vascular collateral status and the approximate size of the irreversibly damaged and the still salvageable brain parenchyma (penumbra concept).
Compared to MRI, a CT stroke protocol brings clear time and logistical advantages (faster data acquisition, wider availability, less effort, better patient monitoring), in line with the motto “time is brain”. Many stroke centers are already using such stroke CT protocols clinically. Direct comparison studies between CT and MRI perfusion provided comparable results [5], but so far valid multicenter comparisons are lacking due to insufficient standardization of the evaluation algorithms/postprocessing [6].
In the following, the possibilities of neuroradiological diagnostics in acute stroke will be presented and underlined with case examples. In addition, we comment on possible sources of error and differential diagnostics.
Native T
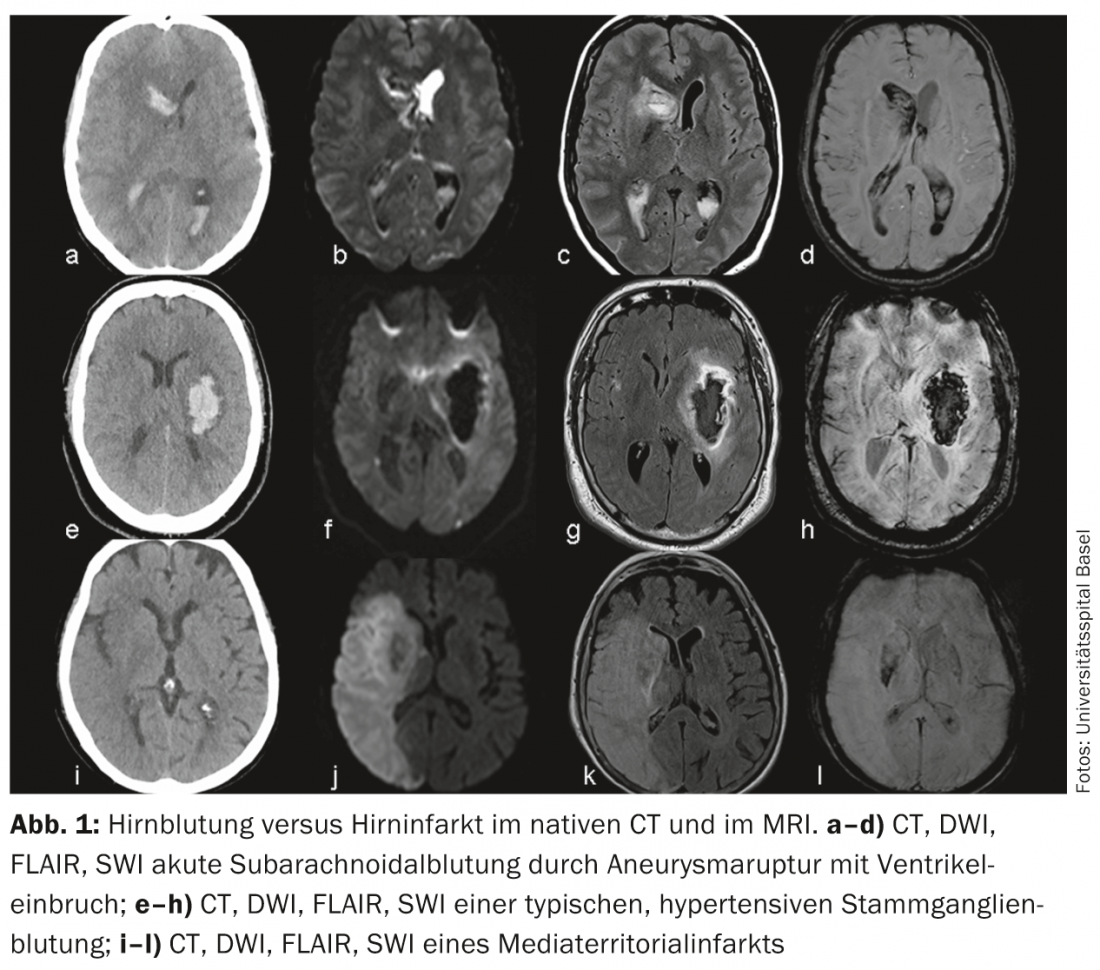
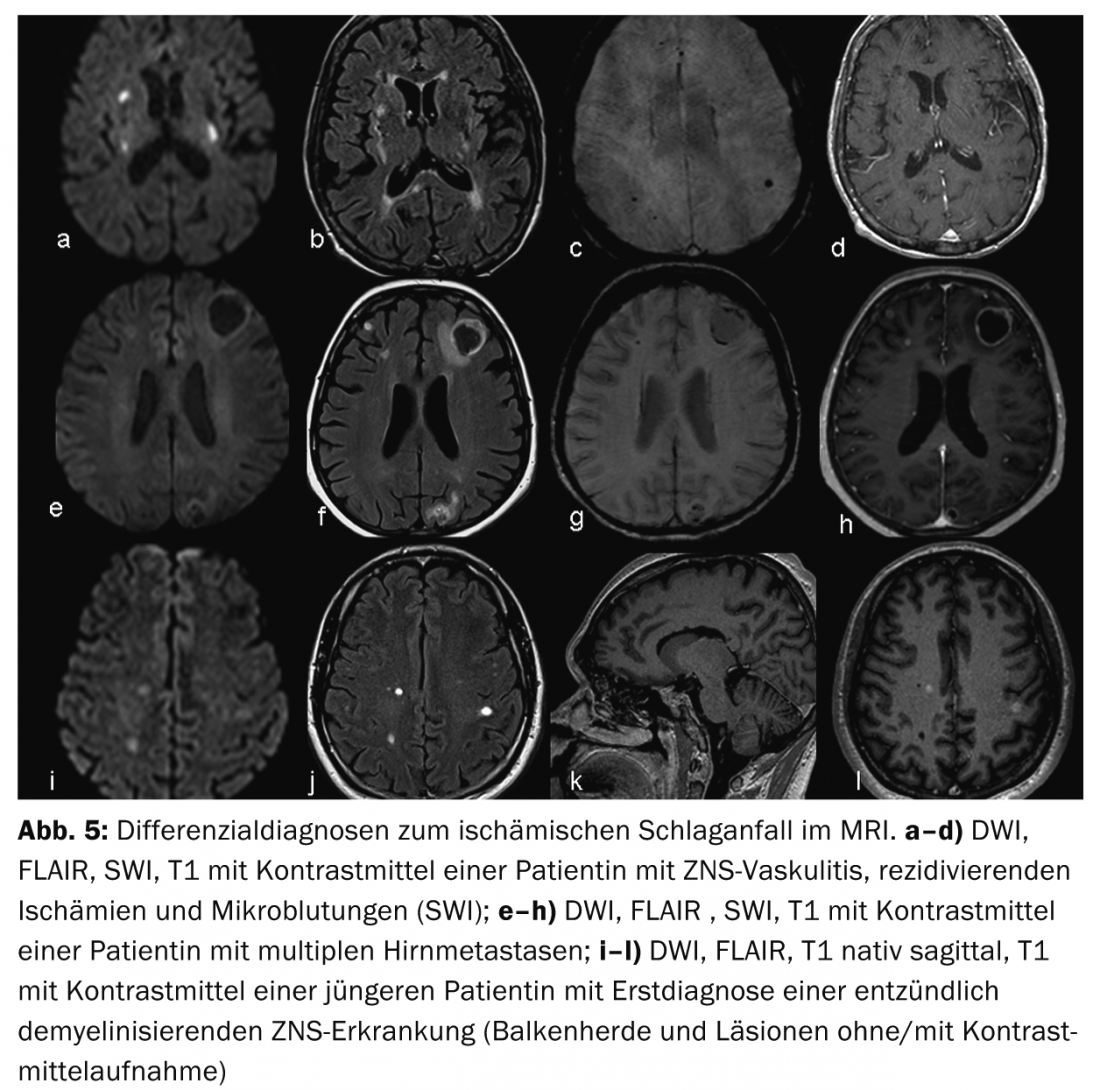
Cerebral infarction versus cerebral hemorrhage: The most common differential diagnosis of ischemic stroke is intracranial hemorrhage. A distinction can be made between subarachnoid (aneurysmal) and intraparenchymal (hypertensive and nonhypertensive) hemorrhage. Detection or exclusion of cerebral hemorrhage is rapid and reliable with native CT, but can be done equally well with modern sequences (SWI, DWI, FLAIR) and a little more effort with MRI (Fig. 1).
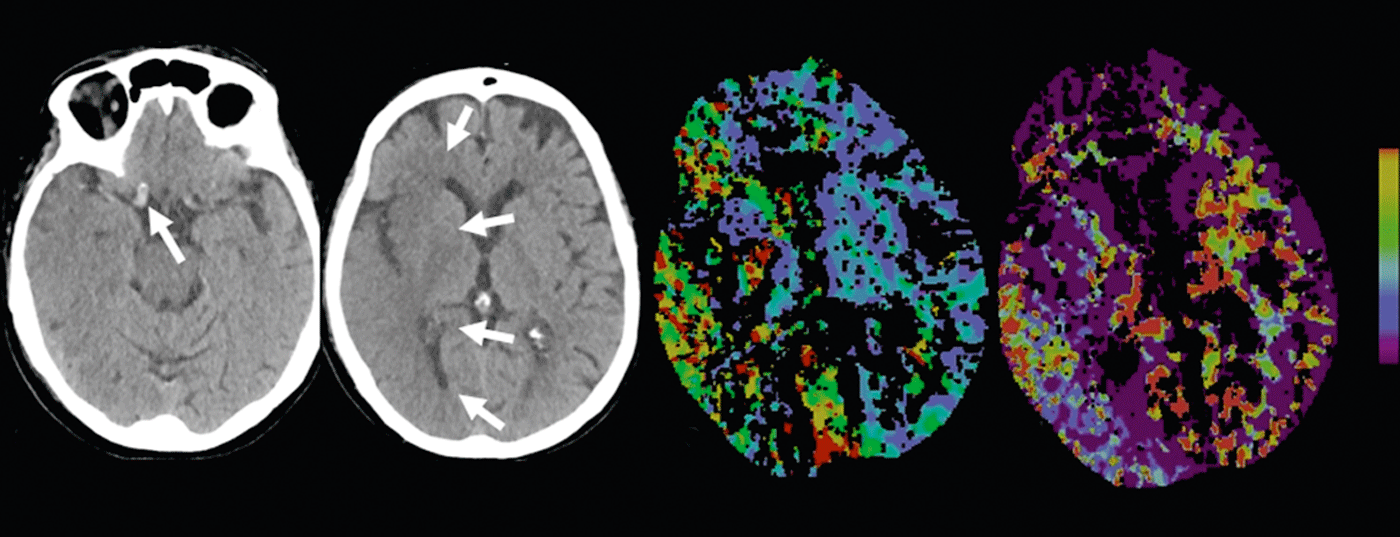
Cerebral infarction: Native CT can reveal so-called early signs of infarction. For example, gray matter density reductions with corticomedullary dedifferentiation due to infarct edema – or the hyperdense artery sign due to density increase with intraluminal thrombus (Figs. 2 and 3).
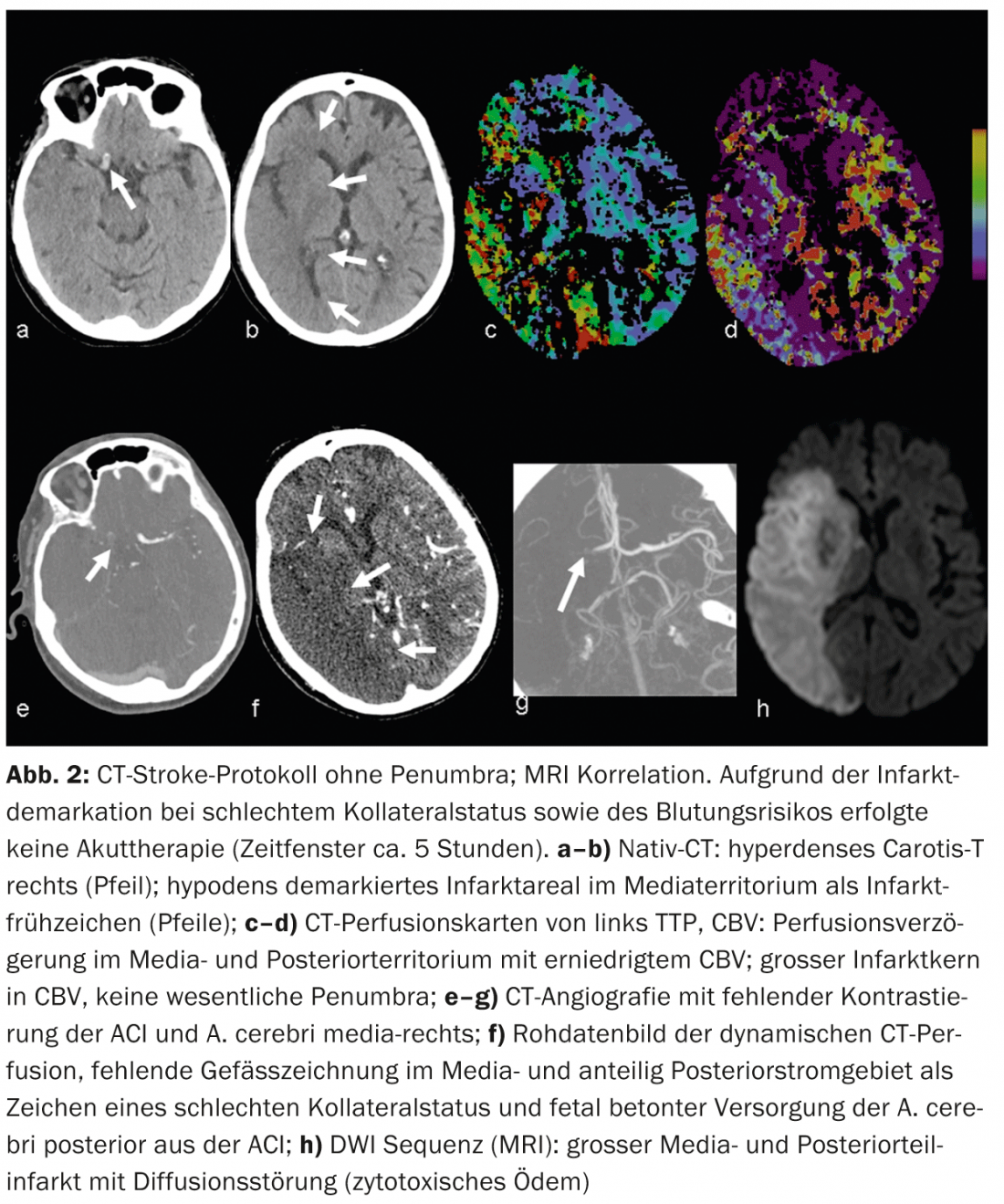
According to the literature, the sensitivity in detecting early signs of infarction varies between 12 and 92% and depends mainly on the duration of ischemia. Infarct areas demarked on native CT are highly specific for irreversible parenchymal damage and are prognostically significant [7]. For example, if more than one-third of the mediastinum shows infarct demarcation, the patient’s risk of hemorrhage increases with iv thrombolytic therapy.
MRI
Best method to visualize ischemic parenchymal damage is MRI, which can visualize infarction within minutes, due to cytotoxic edema. This leads to a reduction of Brownian molecular motion and thus to the detectable diffusion disorder in DWI, which corresponds to the irreversibly damaged infarct core. MRI perfusion studies may reveal perfusion deficits that also infarct (“tissue at risk”) in the absence of reperfusion. This diffusion-perfusion mismatch (penumbra concept) has been intensively studied in the last decade and considered an attractive concept for patient selection for reperfusion therapies [5]. MR angiographies can also be used to image the patient’s intracranial and extracranial vascular status and reveal vascular occlusions.
In routine clinical practice, few hospitals can provide MRI for acute stroke diagnosis; moreover, the examination time is longer, the logistics are more complex, and ultimately, it can only be established as standard acute diagnosis if the infrastructure is very good.
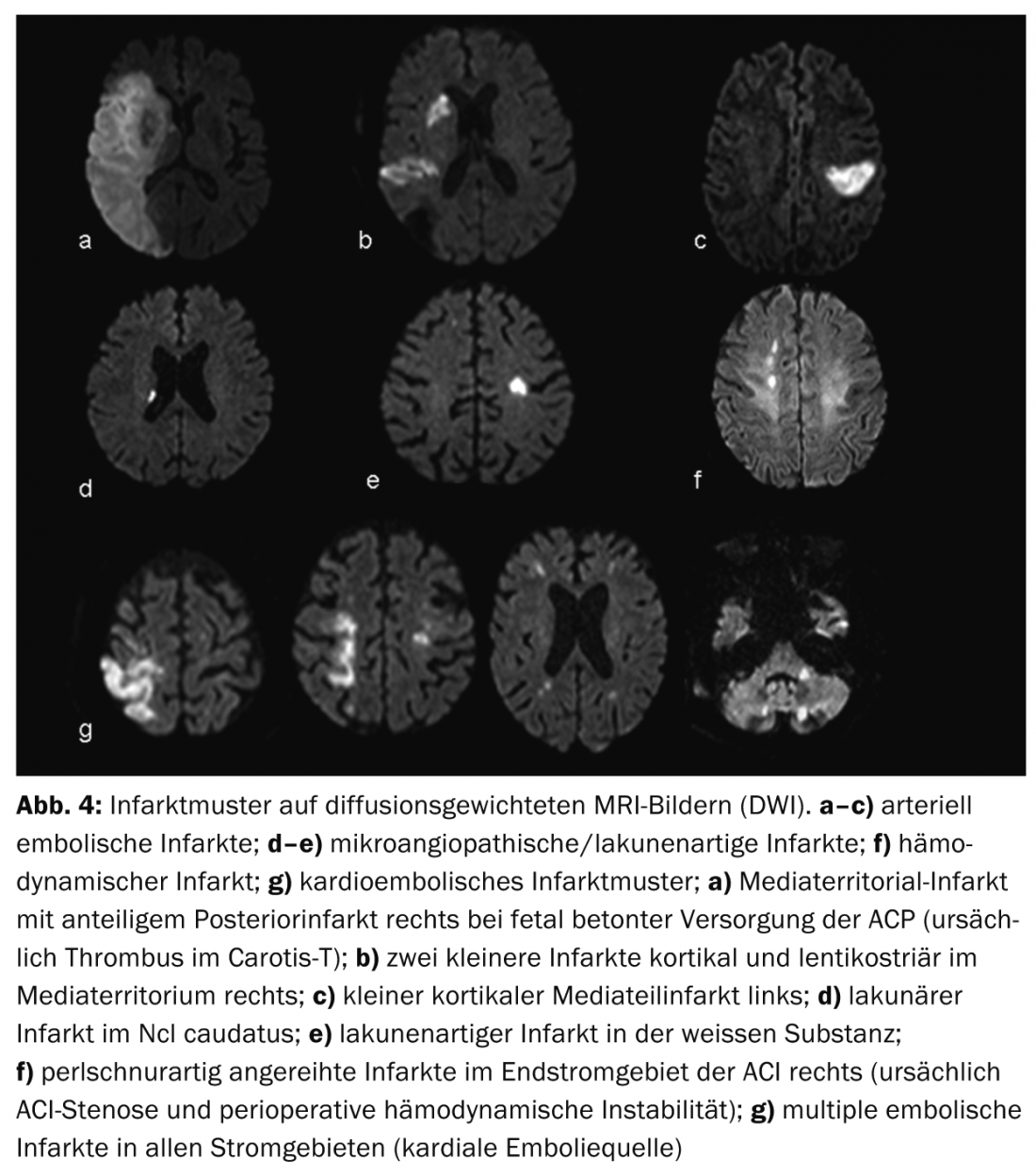
Advantages of MRI are better visualization of infarct patterns and thus better assessment of infarct etiology (Fig.4) as well as better differentiation of differential diagnoses, so-called stroke mimics, e.g. vasculitis, tumor or inflammatory demyelinating CNS diseases (Fig.5). The penumbra concept is particularly useful for therapy decisions in cases of strokes with an unclear time window (so-called “wake-up stroke”).
Modern CT Stroke Protocol
Using modern CT technology, the sensitivity for detecting ischemic cerebral infarction can be significantly increased by adding KM-enhanced CT perfusion and CT angiography.
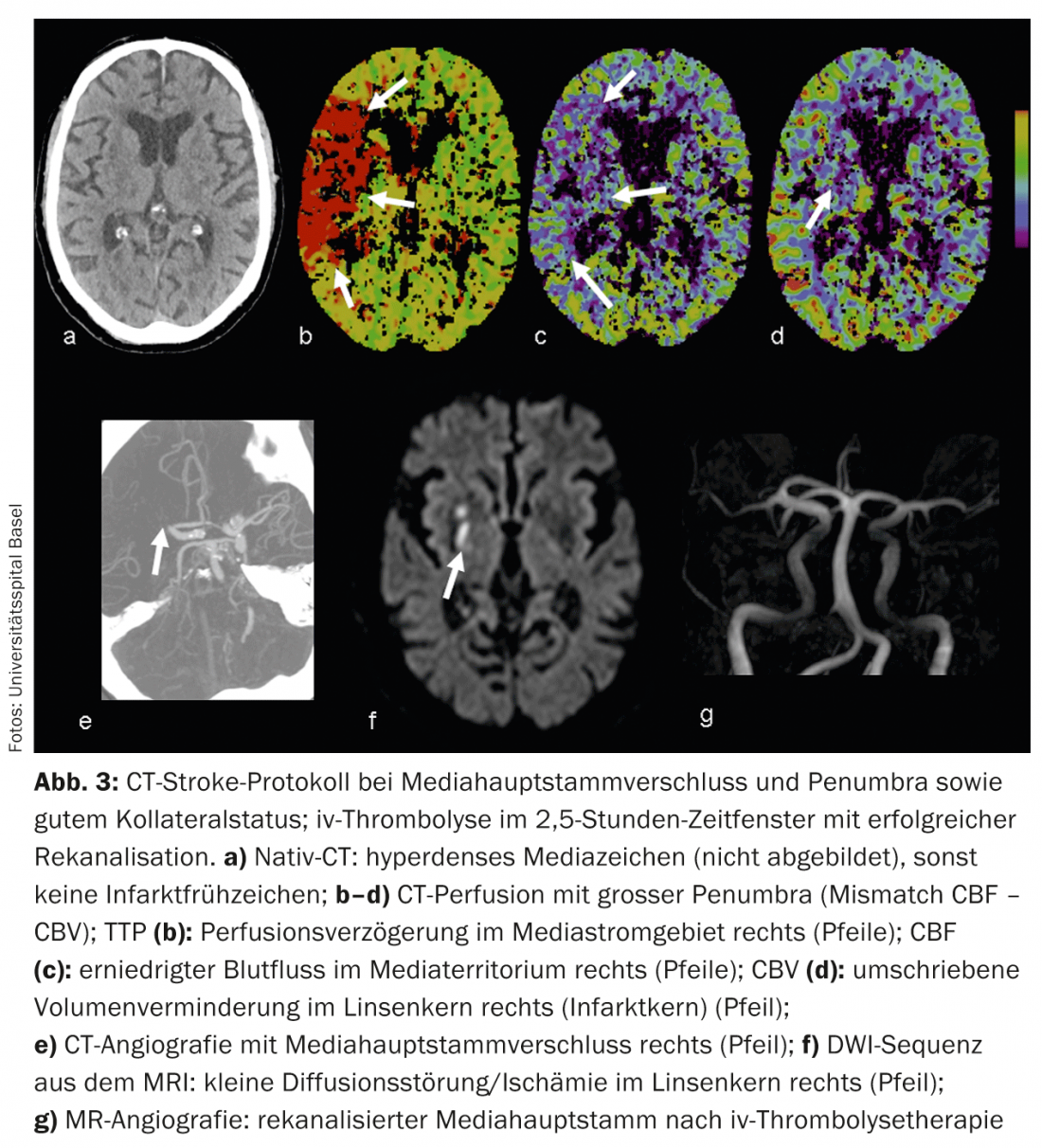
CTA: Contrast-enhanced CTA reliably depicts vascular anatomy, pathology, and collateralization. Vascular collateral status has increasingly become a focus of clinical and scientific interest. Individually, collateral status may have considerable influence on the timing of the ischemic cascade. Intact collateralization is prognostically significant [8]; in addition to the adage “time is brain”, one also speaks of “collateralization is brain”. Collateral supply to the brain parenchyma occurs via the anatomically individually variant circulus arteriosus Willisi as a primary network, and also via leptomeningeal anastomoses between the different vascular territories as a secondary network. The extent and number of anastomoses as well as their compensatory capacity are individually variable [9]. A patient’s collateral status may warrant mechanical recanalization therapy outside the 4.5-hour time window. By knowing the individual collateral status, patients can be better selected for different types of therapy (Figs. 2 and 3).
CTP: CT perfusion is a dynamic CT scan after administration of an iv contrast bolus over a period of approximately 45 seconds. Complex deconvolution algorithms transfer the data into functional parameter maps. CBV denotes cerebral blood volume (physiologically 4-5 ml/100 g brain mass), CBF denotes cerebral blood flow, MTT denotes mean transit time as time interval between arterial inflow and venous outflow, and TTP denotes time to peak as time interval between bolus injection and maximal contrast peak. The evaluation of the raw data is semi-automated and fast. According to the mismatch concept, the evaluation of the functional maps allows a statement about the size of the perfusion defect (“tissue at risk”) and the irreversibly damaged infarct core. Thus, the estimation of a penumbra is also possible in CT within a few minutes.
In acute ischemia, cerebral blood flow decreases. Due to the reactive expansion of the capillary bed – with initially intact autoregulation – the blood volume initially remains stable. If autoregulation decompensates, especially in poor collateral status, CBV decreases [10]. Areas with CBV decrease correspond to irreversibly infarcted brain parenchyma and correlate well with the diffusion-impaired area on MRI (Fig.2 and 3). Areas with normal CBV but impaired MTT or CBF correspond to the “tissue at risk”, the potentially salvageable tissue (Fig.3). Sensitivity for infarct detection is significantly higher for CT perfusion (68-83%) compared with native CT alone (19-45%) or native CT plus CTA (up to 58%), each depending on infarct size [2]. False-negative CT perfusion findings are found in small/lacunar infarcts or in infarcts outside the examined brain section, although modern multislice scanners can now cover almost the entire cerebrum in one examination.
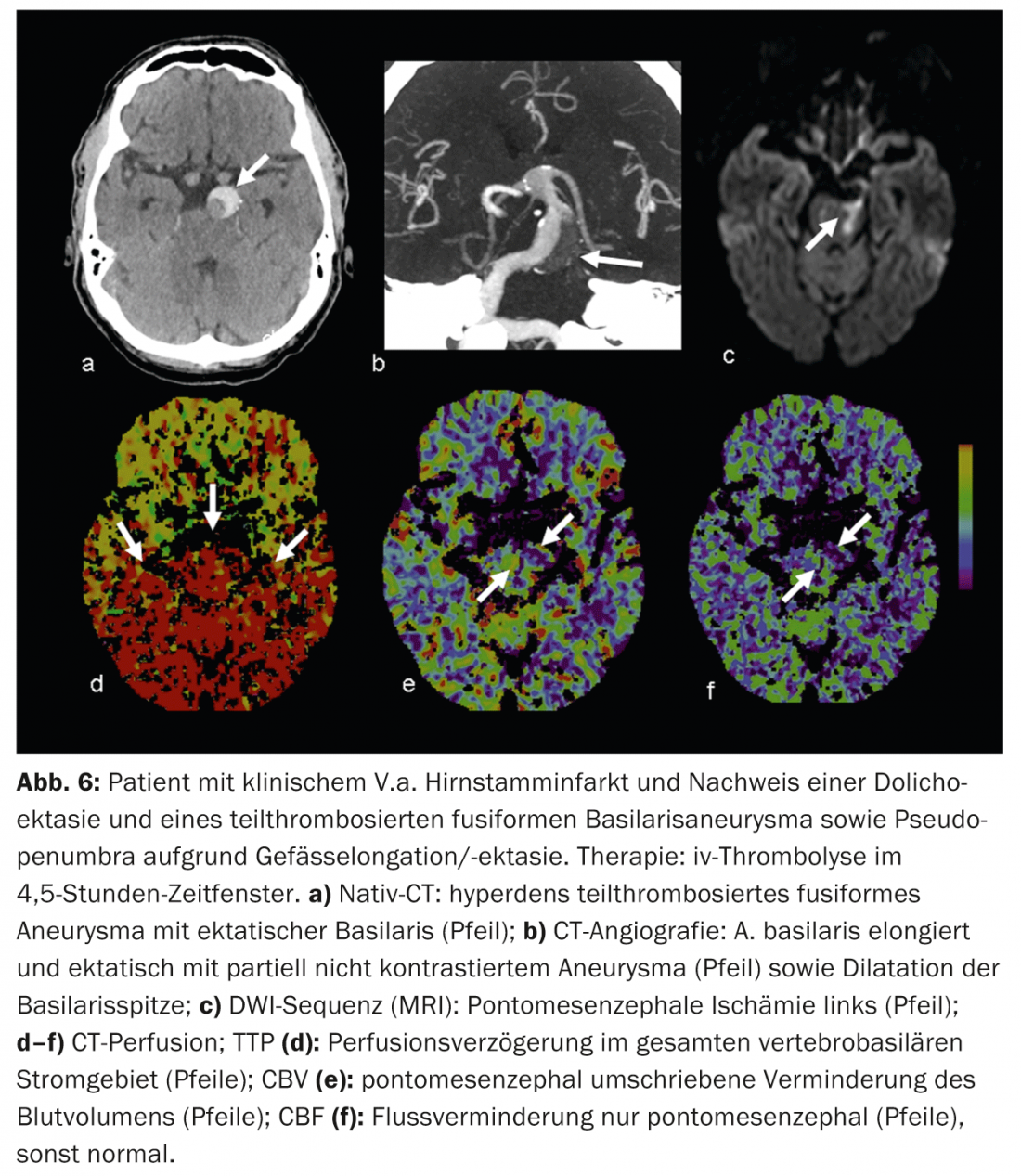
False positive findings arise, for example, from concomitant cardiovascular diseases that can lead to chronic perfusion delay and should not be confused with “pseudopenumbra,” for example, reduced cardiac output function in heart failure, higher-grade upstream vascular stenoses (usually ACI stenoses), occlusions, or even dolichoectasias (e.g., posterior circulation) (Fig. 6).
Johanna M. Lieb, MD
Literature:
- Hacke W, et al: Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317-1329.
- Campbell BCV, et al: CT perfusion improves diagnostic accuracy and confidence in acute ischemic stroke. J Neurol Neurosurg Psychiatry 2013; 84: 613-618.
- Mokin M, et al: Endovascular treatment of acute ischemic stroke: the end or just the beginning? Neurosurg Focus 2014 Jan; 36(1): 1-10.
- Broderick JP, et al: Interventional Management of Stroke (IMS) III Investigators. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013 Mar 7; 368(10): 893-903.
- Campbell BC, et al: Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke 2012 Oct; 43(10): 2648-2653.
- Dani KA, et al: Systematic review of perfusion imaging with computed tomography and magnetic resonance in acute ischemic stroke: heterogeneity of acquisition and postprocessing parameters: a translational medicine research collaboration multicentre acute stroke imaging study. Stroke 2012 Feb; 43(2): 563-566.
- Nabavi DG, et al: MOSAIC: Multimodal Stroke Assessment Using Computed Tomography: novel diagnostic approach for the prediction of infarction size and clinical outcome. Stroke 2002; 33: 2819-2826.
- Nambiar V, et al: CTA-Collateral Status and Response to Recanalization in Patients with Acute Ischemic Stroke. AJNR Am J Neuroradiol 2013 Dec 26. [Epub ahead of print]
- Love child DS: Collateral circulation. Stroke 2003; 34: 2279-2284.
- Ahlhelm F, et al: Focused neuroradiological diagnosis in acute cerebral stroke. Therapeutische Umschau 2012; 69(9): 6.1-6.6.
InFo Neurology & Psychiatry 2014; 12(2): 8-13.