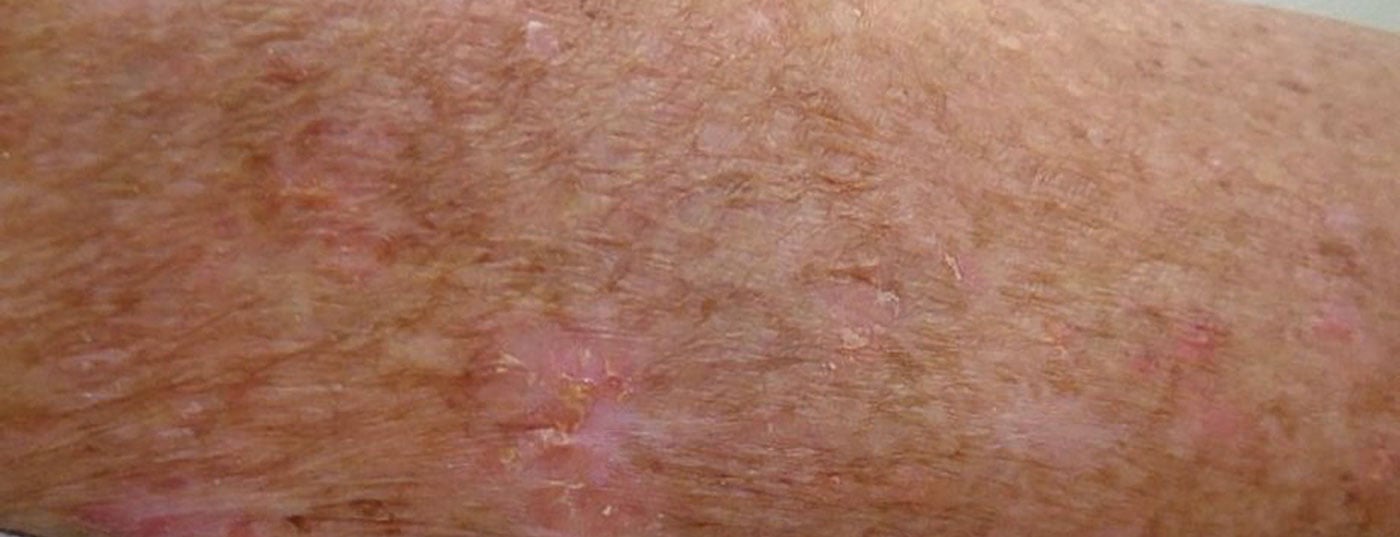
If actinic keratoses form on sun-damaged skin, treatment is recommended to reduce the risk of progression to carcinoma. The spectrum of topical treatment options has recently expanded. First, the novel active ingredient tirbanibulin received EMA approval a few months ago. Tirbanibulin inhibits tubulin polymerization, thereby arresting the cell cycle. Second, a new 4% 5-fluorouracil formulation proved to be equally effective as the 5% preparation.
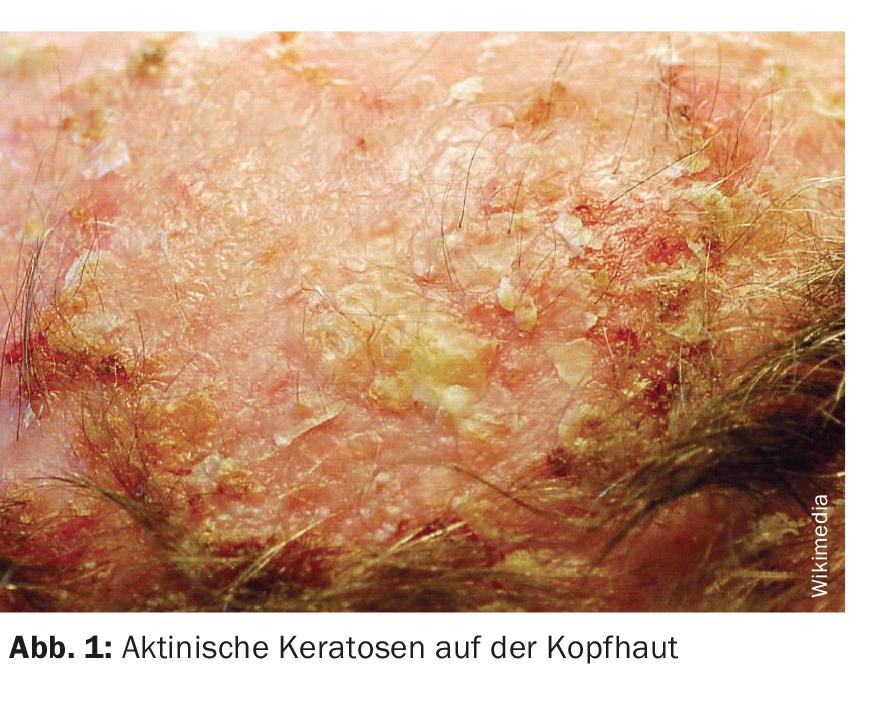
Actinic keratoses (Fig. 1) are the most common dermatoses on chronically sun-damaged skin, especially in individuals with fair skin types. Prevalence increases significantly from the sixth decade of life. Actinic keratoses are defined as intraepidermal proliferation of atypical keratinocytes on UV-damaged skin with the potential to progress into invasive cutaneous squamous cell carcinoma [1]. Predilection sites of actinic keratoses are face, ears and scalp. In European countries, the prevalence in those over 60 years of age is between 20-35% [2,3]. The UV-induced lesions develop into rough, keratinized, and scaly patches that may be skin-colored or red. Even though they are usually benign: in up to 16% of cases, actinic keratoses can develop into squamous cell carcinoma, which is the second most common malignant epithelial skin tumor after basal cell carcinoma (box) [4,5]. A multilevel scaling has been proposed to describe the transition from actinic keratoses to squamous cell carcinoma [6]. The progressive stages of keratinocytic intraepidermal neoplasia (KIN) have been classified into three stages [6]. In KIN I, atypical keratinocytes are found in the lower third of the epidermis. This stage may develop into lesions occupying the lower two-thirds of the epidermis (KIN II) and subsequently cover the full thickness of the epidermis (KIN III). In a paper published in JEADV, actinic keratoses (AK) were classified as premalignant and/or precancerous, with only KIN III/AK III considered in situ squamous cell carcinoma [10].

4% 5-FU cream: comparable efficacy to 5% preparation
On the occasion of this year’s annual congress of the Arbeitsgemeinschaft Dermatologische Onkologie (ADO), Prof. Dr. med. Thomas Dirschka, CentroDerm, Wuppertal (D), gave an up-to-date overview of treatment options for actinic keratosis [7]. In addition to Efudix® Cream 5%, Tolak® Cream 4%, a new topical preparation containing 40 mg/g 5-fluorouracil (5-FU), is available [7]. This has added another therapeutic option to the spectrum of therapeutic alternatives to field treatment. Despite lower dosage, Tolak® showed comparable efficacy. “It can be used in all locations and the maximum applicability is 500 cm2, so this is really something for large areas,” said Prof. Dirschka [7]. In a multicenter, double-blind, vehicle-controlled study (n=841), once-daily use of 5-FU 4% cream was compared with twice-daily use of 5-FU 5% cream and vehicle [8]. After four weeks, the two treatment groups showed comparable response: 80.5% of patients with 4% 5-FU versus 80.2% of patients with 5% 5-FU had 75% fewer lesions. Whereas the 40 mg/g cream had been applied 1× daily, the 5% 5-FU cream had been applied 2× daily. The new, lower-dose formulation thus showed a comparable healing rate to the previous standard therapy with only 1× daily application. Optimized tolerability with less severe skin reactions was associated with a lower rate of treatment discontinuation (10.1% for 4% 5-FU vs. 14.9% for 5% 5-FU) [8].
Tirbanibulin: significant reduction in Anzal lesions with little irritation.
The mode of action of tirbanibulin (Klisyri®), an inhibitor of tubulin polymerization, is based on blockade of the intracellular protein tyrosine kinase Src, which is increasingly expressed in AK and plays a role in progression to PEK [1]. The preparation with the novel active substance is applied 1× daily for 5 days, to max. 25 cm2 skin area. The EMA approval was based on the results of two double-blind, vehicle-controlled phase III studies [9]. Subjects (n=702) applied either tirbanibulin ointment 1% (10 mg/g) or a vehicle preparation to lesions on the face or scalp on five days. Two parallel studies were conducted. Complete healing at day 57 was achieved by 44% of tirbanibulin-treated patients in one study versus 5% in the vehicle-treated group (p<0.001). In the second study, complete clearance was observed in 54% vs. 13% of patients (p<0.001). In addition to the primary endpoint, the main secondary endpoint was also met: significantly more patients achieved 75% healing 57 days after baseline under verum treatment than in the control group: 68% vs. 16% (p<0.001) in the first study and 76% vs. 20% (p<0.001) in the second study, respectively [9]. In summary, tirbanibulin not only showed a significant reduction in the number of lesions, but also convinced with a low extent of side effects.
Congress: Working Group Dermatologic Oncology 2021
Literature:
- Borik-Heil L, Geusau A: hautnah 2021; 20: 45-55.
- Ferrándiz C, et al: Actas Dermosifiliogr 2016; 107(8): 674-680.
- Eder J, et al: Br J Derm 2014; 171(6): 1415-1421.
- Stockfleth E: JEADV 2017; 31 (2): 8-11.
- Trautinger F, Beichl-Zwiauer V: Österreichische Ärztezeitung 13/14, July 15, 2021 (online, last accessed Sept. 19, 2021).
- AWMF: S3-Leitlinie: Aktinische Keratose und Plattenepithelkarzinom der Haut. 2020. www.awmf.org, (last accessed 19.09.2021)
- Dirschka T: Non-melanocytic skin tumors – Current status in diagnostics and therapy of actinic keratosis, Prof. Dr. med. Thomas Dirschka, German Skin Cancer Congress, 08.-11.09.2021.
- Dohill MA: J Drugs Dermatol 2016; 15 (10): 1218-1224.
- Blauvelt A, et al: N Engl J Med 2021; 384: 512-520.
- Fernandez-Figueras MT, et al: JEADV 2015; 29(5): 991-997.
- Smit P, et al: JEADV 2013; 27(6): 667-671.
- Swissmedic Journal 03/2021, www.swissmedic.ch, (last accessed Oct. 22, 2021).
- Swissmedic Journal 01/2021, www.swissmedic.ch, (last accessed Oct. 22, 2021).
- University Hospital Zurich, www.usz.ch/krankheit/aktinische-keratose, (S3 guideline) www.awmf.org/uploads/tx_szleitlinien/032-022OLl_S3_Aktinische_Keratosen-Plattenepithelkarzinom-PEK_2020-04.pdf, (last accessed Oct. 22, 2021).
HAUSARZT PRAXIS 2021; 16(12): 40-41 (published 12/14-21, ahead of print).