Actinic keratoses are the most common dermatoses on chronically sun-damaged skin, especially in people with fair skin types. Prevalence increases significantly from the sixth decade of life. The spectrum of lesion-targeted therapies has recently expanded to include a topical treatment option – a novel agent inhibits tubulin polymerization, thereby arresting the cell cycle. In field treatment, a 4% 5-fluorouracil cream has been shown to be comparably effective to the 5% preparation.
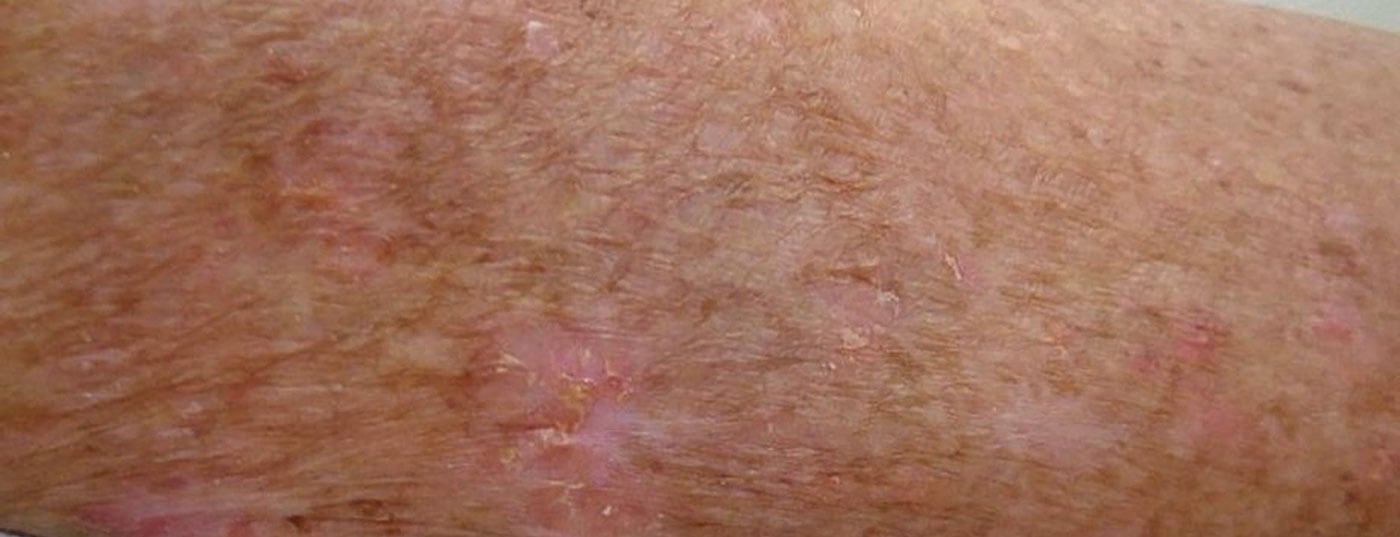
Actinic keratoses are defined as intraepidermal proliferation of atypical keratinocytes on UV-damaged skin with the potential to progress into invasive cutaneous squamous cell carcinoma [1]. Predilection sites are face, ears and scalp. In European countries, the prevalence in those over 60 years of age is between 20-35% . The UV-induced lesions of actinic keratosis develop into rough, keratinized, and scaly patches that may be skin-colored or red. Even though they are usually benign: in up to 16% of cases they can develop into squamous cell carcinoma, which is the second most common malignant epithelial skin tumor after basal cell carcinoma [4,5]. Regarding risk of progression, a multilevel scaling has been proposed to describe the transition from actinic keratosis (AK) to squamous cell carcinoma (PEK) (Box ) [6].
Prof. Dr. med. Thomas Dirschka, CentroDerm, Wuppertal (D), gave an up-to-date overview of treatment options for actinic keratosis on the occasion of this year’s annual congress of the Arbeitsgemeinschaft Dermatologische Onkologie (ADO) [7].
5-fluorouracil: lower-dose preparation with comparable efficacy
In addition to Efudix cream 5%, Tolak® cream 4%, a new topical preparation containing 40 mg/g 5-fluorouracil (5-FU), is available* [7]. This has added another therapeutic option to the spectrum of therapeutic alternatives to field treatment. Despite lower dosage, Tolak® showed comparable efficacy. “It can be used in all locations and the maximum applicability is 500 cm2, so this is really something for large areas,” said Prof. Dirschka [7]. In a multicenter, double-blind, vehicle-controlled study (n=841), once-daily use of 5-FU 4% cream was compared with twice-daily use of 5-FU 5% cream and vehicle [8]. After four weeks, the two treatment groups showed comparable response: 80.5% of patients with 4% 5-FU versus 80.2% of patients with 5% 5-FU had 75% fewer lesions. Therapy with the 40 mg/g cream was used only 1× daily, and the 5% 5-FU cream was used 2× daily. The new, lower-dose formulation thus showed a comparable healing rate to the previous standard therapy with only 1× daily application. Optimized tolerability with less severe skin reactions was associated with a lower rate of treatment discontinuation (10.1% for 4% 5-FU vs. 14.9% for 5% 5-FU) [8].
* Tolak®: Swissmedic approval 02.03.2021 [12]
|
Risk of progression of actinic lesions The progressive stages of keratinocytic intraepidermal neoplasia (KIN) have been classified into three stages [6]. In KIN I, atypical keratinocytes are found in the lower third of the epidermis. This stage may develop into lesions that cover the lower two-thirds of the epidermis (KIN II) and subsequently cover the full thickness of the epidermis (KIN III). In a paper published in JEADV, actinic keratoses (AK) were classified as premalignant and/or precancerous, with only KIN III/AK III considered in situ squamous cell carcinoma [10]. The absolute risk of head AK lesion developing into squamous cell carcinoma within 16-34 months is reported to be 0.42 [11]. |
Tirbanibulin: significant reduction in “lesion count” with little irritation.
The mode of action of tirbanibulin (Klisyri®), an inhibitor of tubulin polymerization, is based on blockade of the intracellular protein tyrosine kinase Src, which is increasingly expressed in AK and plays a role in progression to PEK [1]. The preparation with the novel active substance is applied 1× daily for 5 days, to max. 25 cm2 skin area. The EMA approval** is based on the results of two double-blind, vehicle-controlled Phase III studies [9]. Subjects (n=702) applied either tirbanibulin ointment 1% (10 mg/g) or a vehicle preparation to lesions on the face or scalp on five days. Two parallel studies were conducted. Complete healing at day 57 was achieved by 44% of tirbanibulin-treated patients in one study versus 5% in the vehicle-treated group (p<0.001). In the second study, complete clearance was observed in 54% vs. 13% of patients (p<0.001). In addition to the primary endpoint, the main secondary endpoint was also met: significantly more patients achieved 75% healing 57 days after baseline under verum treatment than in the control group: 68% vs. 16% (p<0.001) in the first study and 76% vs. 20% (p<0.001) in the second study, respectively [9]. In summary, tirbanibulin not only showed a significant reduction in the number of lesions, but also convinced with a low extent of side effects.
** Tirbanibulin: Swissmedic new registration 14.01.2021 [13]
Congress: Working Group of Dermatologic Oncology (ADO)
Literature:
- Borik-Heil L, Geusau A: hautnah 2021; 20: 45-55.
- Ferrándiz C, et al: Actas Dermosifiliogr 2016; 107(8): 674-680.
- Eder J, et al: Br J Derm 2014; 171(6): 1415-1421.
- Stockfleth E: JEADV 2017; 31 (2): 8-11.
- Trautinger F, Beichl-Zwiauer V: Österreichische Ärztezeitung 13/14, July 15, 2021 (online), (last accessed Sept. 19, 2021).
- AWMF: S3-Leitlinie: Aktinische Keratose und Plattenepithelkarzinom der Haut. 2020. www.awmf.org, (last accessed 19.09.2021)
- Dirschka T: Non-melanocytic skin tumors – Current status in diagnostics and therapy of actinic keratosis, Prof. Dr. med. Thomas Dirschka, German Skin Cancer Congress, 08.-11.09.2021.
- Dohill MA: J Drugs Dermatol 2016; 15 (10): 1218-1224.
- Blauvelt A, et al: N Engl J Med 2021; 384: 512-520.
- Fernandez-Figueras MT, et al: JEADV 2015; 29(5): 991-997.
- Smit P, et al: JEADV 2013; 27(6): 667-671.
- Swissmedic Journal 03/2021, www.swissmedic.ch, (last accessed Sept. 19, 2021).
- Swissmedic Journal 01/2021, www.swissmedic.ch, (last accessed Sept. 19, 2021).
DERMATOLOGY PRACTICE 2021; 31(5): 50