In rheumatoid arthritis, the immune system attacks the synovial membrane of the synovium. If left untreated, there is a risk of gradual joint destruction. DMARDs are therefore used in severe courses. Omics analyses of individual cell types provide new insights into disease progression. Among other things, the focus is on research into T-cell activity and different types of tissue macrophages as a basis for new treatment strategies. Current findings and study results were reported at the annual meeting of the German Society of Rheumatology (DGRh).
For a differentiated understanding of disease and derived treatment options, research into the complex molecular events that occur at the cellular level is very informative. Advances in high-throughput technologies (“omics”) have made observations at the genomic, transcriptomic and regulatory levels of various biological molecules possible (box). The further development of therapeutic approaches against rheumatoid arthritis has benefited greatly from the findings of basic research in recent decades. “Rheumatologists are now well able to influence the course of the disease by injecting or infusing various biological antibodies ranging from abatacept to rituximab,” explains the spokesman of the Rheumatism Competence Network Working Group, Prof. Ulf Wagner, MD, of Leipzig University Hospital [1]. Most recently, synthetic drugs such as baricitinib or tofacitinib, which can be taken as tablets, have been added. “The new agents make treatment more tolerable for many patients,” the expert said.

Further develop the therapeutic principle of modulating T-cell activity
Activation of autoreactive T cells is of central pathogenetic importance in autoimmune diseases such as rheumatoid arthritis. They are involved in the inflammatory cascade by influencing other immune cells and producing a number of proinflammatory cytokines. Based on these findings, various concepts for therapeutic blockade of T cells have been developed. These aim to either largely eliminate T cells from the disease process or to influence their function in such a way that their pathogenic effects are slowed down. Abatacept (Orencia®) is a modulator of T-cell activity that has been used therapeutically for some time. The mechanism of action is to specifically block the CD28-CD80/CD86 pathway [2,3]. CD28 is one of the most prominent costimulatory molecules, expressed by about half of T cells. CD28 has two ligands, CD80 and CD86, which are expressed on the surface of various antigen-presenting cells in an activation-dependent manner. In addition, “cytotoxic T-lymphocyte-associated antigen 4” (CTLA-4) is also expressed on T cells in an activation-dependent manner and also binds to CD80 and CD86. However, unlike CD28, CTLA-4 slows T-cell activation via inhibition of proliferation and cytokine production and thus can be considered an antagonist of CD28.
Modulation of harmful T cells is also the focus of a new treatment strategy currently being explored. “We currently assume that at the beginning of the disease there is a loss of self-tolerance, which normally prevents T cells from attacking the body’s own cells in the synovial membrane,” says Prof. Ulf Wagner, MD [1]. “The T cells begin to attack the synovial cells, and they cause the B cells to produce antibodies, of which rheumatoid factor is the best-known example.” According to the expert, the omics studies show that the metabolism of T cells changes completely in the course of the disease. For example, the energy supply is switched from glycolysis to the pentose phosphate pathway. “These altered metabolic processes may provide completely new possibilities for therapeutically influencing chronic inflammatory autoimmune diseases,” explains Prof. Wagner. “Our goal should be to turn the ‘bad’ T cells into ‘good’ T cells and stop the disease process right at the beginning.” The future will show whether this treatment approach will become established and lead to the market launch of corresponding active substances.
Tissue macrophages as part of the inflammatory cascade.
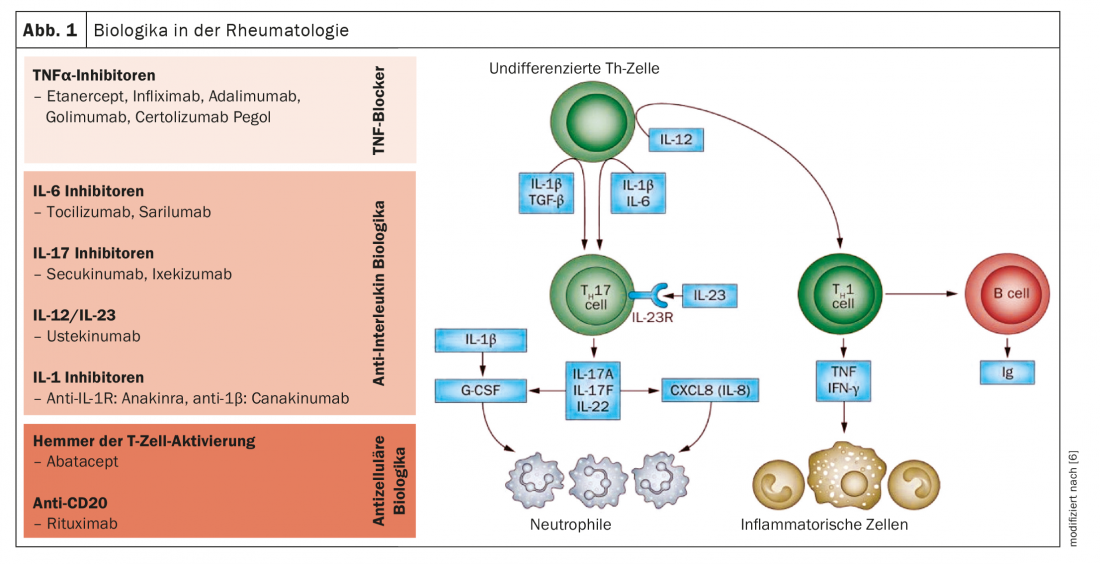
The biological antibodies and synthetic drugs approved to date block cytokines or their receptors, which are released by the cells of the immune system as part of the inflammatory processes (Fig. 1 and 2) . Basic research has now turned to the different inflammatory cells that produce these cytokines. Newer omics methods such as single cell RNA sequencing, ribosome profiling, or mass spectrometry provide insight into individual cells for the first time. “The studies show which cell groups behave differently in rheumatism patients than in healthy individuals and which are therefore likely to be involved in the disease process,” explains Prof. Wagner [1]. By combining different omic technologies, one can get a more complete picture of gene expression, protein quantity, or various intracellular processes. One of the central players in rheumatoid arthritis is tissue macrophages, which are also present in the synovial membrane in healthy individuals. “Research wants to clarify what makes these cells release the inflammatory cytokines and how this could be prevented,” the expert said.
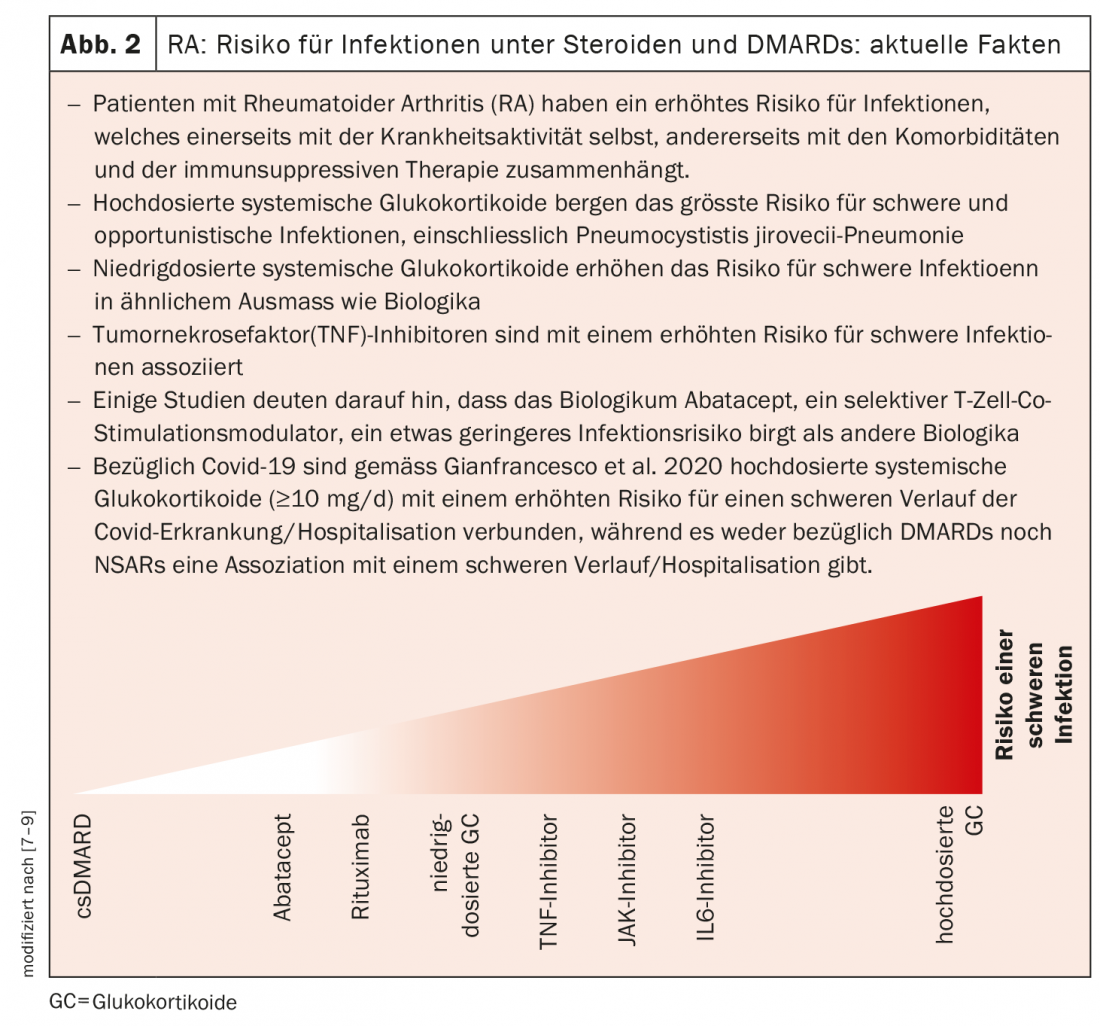
MerTK-negative and CD206-negative tissue macrophages as targets.
For example, basic researchers have found that there are two groups of tissue macrophages that are distinguished by the surface markers MerTK and CD206. A recent study published in Nature Medicine found that MerTK-negative and CD206-negative tissue macrophages produce a number of pro-inflammatory cytokines and alarmines, promoting inflammatory responses in the synovial membrane [4]. In contrast, MerTK-positive and CD206-positive tissue macrophages appear to inhibit inflammatory responses. These cells are found mainly in patients whose inflammation has completely subsided. Prof. Wagner explains, “The idea for a therapeutic approach would be to use drugs to put the tissue macrophages into a permanent dormant state and thereby stop the disease in the long term.”
Congress: DGRh Annual Conference
Literature:
- “Rheumatoid arthritis: analysis of single cells allows new insights into the disease process – new therapeutic approaches expected”, German Rheumatology Congress, Sept. 16, 2021.
- Swissmedicinfo: Orencia®, www.swissmedicinfo.ch (last accessed Nov. 18, 2021).
- Graninger W, Emminger W, Scheinecker C: J Med Drug Rev 2013; 3: 44-60.
- Alivernini S, et al: Nat Med 2020; 26(8): 1295-1306.
- Rosenstiel P, Franke A, Schreiber S: Predicting and individually treating chronic inflammation. www.systembiologie.de (last call 11/18/2021)
- Mihai C: Rheumatism Workshop, Carmen-Marina Mihai, MD, Sept. 23, 2021; www.usz.ch/app/uploads/2021/09/CMihai_NW-Biologika_RheumaWorkshop_Sept21.pdf (last accessed Nov. 18, 2021).
- Tamborrini G: Risk of infection under DMARD’s and steroids, Weekly 2021, www.rheuma-schweiz.ch (last accessed Nov. 18, 2021).
- Riley TR, et al: Risk for infections with glucocorticoids and DMARDs in patients with rheumatoid arthritis, RMD Open 2021: online.
- Gianfrancesco M, et al: Ann Rheum Dis 2020; 79: 859-866.
InFo PAIN & GERIATry 2021; 3(2): 30-31.
HAUSARZT PRAXIS 2021; 16(12): 20-22