Often, psoriasis patients not only suffer from skin lesions, but are also affected by various comorbidities and stigma. In order to limit the negative impact on physical and psychosocial aspects, it is important in moderate to severe psoriasis to achieve the best possible symptom control early in the course of the disease. The use of modern biologics plays an important role in this.
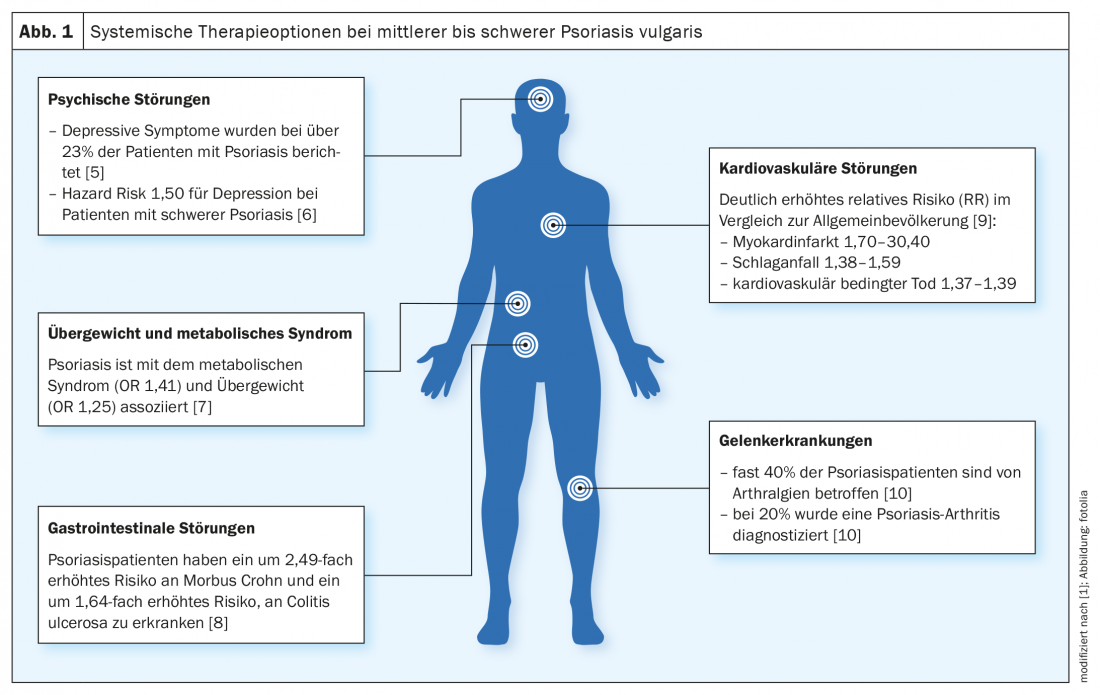
“Psoriasis is not only a disease of the skin, but also a systemic disease,” explains PD Dr. med. Julia-Tatjana Maul, senior physician and head of the psoriasis consultation, University Hospital Zurich, on the occasion of the virtual continuing education event “New Horizons” [1]. Comorbidities often manifest themselves, but stigmatization and compliance also influence well-being and quality of life. The most common comorbid conditions include depression, obesity, metabolic syndrome, inflammatory bowel disease and psoriatic arthritis (Fig. 1) . “And we also know that cardiovascular risk factors play a big role in psoriasis patients,” she said, explaining, “We want to treat not only the skin lesions but also the comorbidities with the new therapeutic options.”
“Reducing the burden of disease and improving quality of life
But what is the best strategy to minimize the cumulative impact of psoriasis? An important factor in containing the burden of disease is timely personalized treatment, for which a broad spectrum of highly effective drugs is now available. It has been shown in many publications that appearance-free skin is a very important factor for the quality of life measurable by the “Dermatology Life Quality Index” (DLQI). “Those patients with a low severity of psoriasis had the best quality of life” [2,3], summarizes PD Dr. Maul. In general, good treatment with a rapid onset of action is desirable, she said. This can also reduce the cumulative impairments due to the disease and thus the comorbidities.
In the past decades, the therapeutic landscape for the systemic treatment of plaque psoriasis has changed significantly, and numerous new drugs have been approved, particularly in the field of biologics. If a psoriasis patient is not achieving treatment goals on conventional systemic therapies, a switch to biologics should be considered. In this context, it is important to coordinate the various treatment options for psoriasis as optimally as possible.
Set common therapy goals
“If there is an imbalance between therapy and physical and psychological comorbidities and stigma, the patient will suffer more from their therapy over the course of their life, and cumulative impairment will increase,” PD Dr. Maul elaborates. Modern systemic agents have great therapeutic potential. “Biologics can achieve better therapeutic outcomes than conventional systemic therapeutics,” she said. In addition to the severity of the psoriasis and any comorbidities present, it also plays a role which previous therapies the patient has already had, or whether the conditions for biologic therapy are met*. The appropriate active ingredient is then selected, the therapy is initiated and the accompanying monitoring is initiated. If there is no sufficient response to therapy after a period of 4-6 months, a dose adjustment or a change in therapy should be made. It is very important to discuss this with the patient and to agree on common treatment goals [1].
* Limitation for the use of biologics in Switzerland: insufficient response to conventional systemic therapy or PUVA (psoralen and UV-A) or contraindications or intolerance to these therapies. [11]
High PASI response rates of risankizumab under long-term treatment.
In the open-label LIMMitless** study, the IL23p19 inhibitor risankizumab showed sustained high efficacy both after switching from ustekinumab or adalimumab and with continuous risankizumab delivery [4]. Study participants received long-term treatment with risankizumab 150 mg every 12 weeks. Among patients randomized to the ustekinumab arm in UltIMMa-1 or UltIMMa-2 (n=199), 172 were included in the LIMMitless trial and switched to risankizumab. The PASI90 response rate# was 83% at week 84 versus 47% at baseline (with ustekinumab). The corresponding PASI75 response rates were 98% vs. 78%, and a substantial 57% achieved PASI 100, compared with only 27% in the ustekinumab arm. The evaluation of the DLQI (Dermatology Life Quality Index) values shows that the quality of life of the patients improved significantly under treatment with the IL23 inhibitor. At week 72, 81% of study participants on risankizumab had a DLQI score of 0 or 1 (LOCF$), a substantial increase from baseline when this score was 50%.
** The LIMMitless study included patients who had completed one of seven phase II/III studies (including UltIMMa-1, UltIMMa-2 and IMMvent) [4].
# observed cases, no imputation of missing values; analyses of PASI response rates using the last observation carried forward (LOCF) and modified non-responder imputation methods showed similar results [4].
$ LOCF = “last observation carried forward”
Overall, IL23 and IL17 inhibitors generally show very good and long-lasting efficacy, PD Dr. Maul said, although there are some differences within these groups. In particular, the IL23 inhibitors, along with the IL17 inhibitors, are also considered very safe drugs, as shown on the one hand by the Real World data from our Swiss Psoriasis Registry SDNTT, but also by the 3-5 year safety data, according to the head of the Psoriasis Consultation Unit at the University Hospital Zurich [1].
Congress: USZ New Horizons 2021
Literature:
- Maul JT: Optimizing Psoriasis Management. PD Julia-Tatjana Maul, MD, USZ New Horizons, 04/15/2021.
- Takeshita J, et al: Patient-reported outcomes for psoriasis patients with clear versus almost clear skin in the clinical setting. JAAD 2014; 71(4): 633-641.
- J-T Maul, et al: Gender and age significantly determine patient needs and treatment goals in psoriasis – a lesson for practice. JEADV 2019; 33(4): 700-708.
- Blair HA: Risankizumab: A Review in Moderate to Severe Plaque Psoriasis. Drugs 2020; 80: 1235-1245.
- Dowlatshahi EA, et al: The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta-analysis.
- J Invest Dermatol 2014; 134(6): 1542-1551.
- Egeberg JP, Thyssen JJ, Wu L, Skov A: Risk of first-time and recurrent depression Risk of first-time and recurrent depression in patients with psoriasis: a population-based cohort study. British Journal of Dermatology 2019; 180(1): 116-121.
- Langan SM, et al: Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol 2012; 132(3 Pt 1): 556-562.
- Vlachos C, et al: Psoriasis and inflammatory bowel disease: links and risks. Psoriasis (Auckl) 2016; 6: 73-92.
- Egeberg A, et al: The role of the interleukin-23/Th17 pathway in cardiometabolic comorbidity associated with psoriasis. JEADV 2020; 34(8): 1695-1706.
- Stern RS: The epidemiology of joint complaints in patients with psoriasis. J Rheumatol 1985; 12(2): 315-320.
- BAG: www.bag.admin.ch (last accessed 07.06.2021)
DERMATOLOGIE PRAXIS 2021; 31(4): 28-29 (published 8/18-21, ahead of print).