The therapy of ALK-positive or ROS1-positive NSCLC has evolved greatly in recent years with the availability of various ALK or ROS1 inhibitors. This CME will summarize and discuss the current ESMO guidelines and key trial data.
With 4300 new diagnoses per year, bronchial carcinoma is one of the most common cancers in Switzerland [1]. The disease is also associated with the highest (21.6%) cancer-related mortality in men and the second highest (15.7%) after breast cancer in women [1]. Non-small cell lung cancer (NSCLC) is present in 85% of patients, most commonly with adenocarcinoma histology (40%). In addition, anaplastic lymphoma kinase (ALK) fusions can be detected in about 5% of tumors and ROS proto-oncogene 1, receptor tyrosine kinase (ROS1) fusions in about 4.5% [2]. Compared with the rest of the population, patients with ALK- or ROS1-positive NSCLC are younger and more often never- or few-smokers [3,4]. Meanwhile, ALK/ROS1 biomarker testing is an established component in the diagnosis of NSCLC and forms a necessary basis for treatment decisions [5]. In patients with metastatic disease (stage IV), treatment is in the palliative setting and aims to improve symptoms, at least maintain quality of life, and prolong overall survival [6]. With the approval of ALK inhibitors of the 1st (crizotinib), 2. (alectinib, ceritinib) and 3rd generation (lorlatinib), therapy has been revolutionized and personalized [7]. This CME will summarize and discuss the current ESMO guidelines and key trial data. The focus is on drugs approved in Switzerland [8].
First-line therapy
The ESMO guidelines recommend the use of crizotinib, alectinib, ceritinib, or brigatinib** in the first line of therapy (Table 1) [5]. In the PROFILE-1014 trial, a significant prolongation of median progression-free survival (PFS) was observed with crizotinib compared to chemotherapy with cisplatin or carboplatin in combination with pemetrexed (10.9 vs. 7.0 months, HR 0.45, p>0.001) [13]. Objective response rates (ORR) were 74% with crizotinib and 45% in the chemotherapy arm [13]. After an observation period of approximately 46 months, median OS had not yet been reached in the crizotinib arm and was 47.5 months with chemotherapy (HR 0.76, p=0.0978). Although crizotinib was numerically superior, there was no statistically significant difference in OS between treatment arms, most likely due to crossover effects or very effective follow-up therapies after progression, respectively [14].
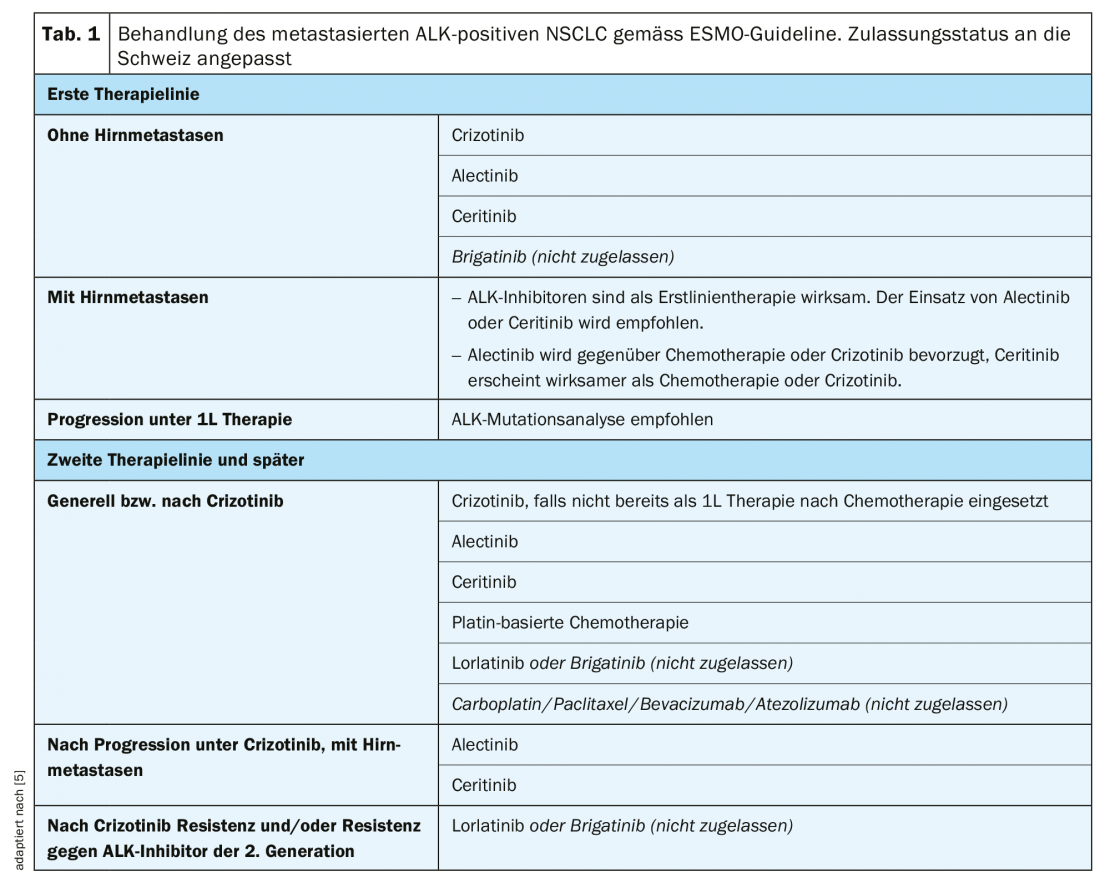
As another option in first-line therapy, ceritinib showed significantly improved median PFS (16.6 vs. 8.1 months, HR 0.55, p<0.00001) compared with platinum-based chemotherapy (cisplatin or carboplatin plus pemetrexed followed by pemetrexed maintenance therapy) in the ASCEND-4 trial [15]. The median OS had not yet been reached with ceritinib and was 26.2 months with chemotherapy (HR 0.73, p=0.056) [15].
The ALEX trial compared the 2nd generation ALK inhibitor alectinib with crizotinib in previously untreated patients [16]. Alectinib showed significantly improved median PFS compared with crizotinib (not reached vs. 11.1 months, HR 0.47, p<0.001) [16]. A similar result was also achieved in the Japanese J-ALEX trial (alectinib, not reached vs. crizotinib, 10.2 months, HR 0.34, p>0.001) [17]. Alectinib therapy was better tolerated in both trials, with fewer interruptions, dose reductions, or discontinuations of therapy than crizotinib [16,17].
Development of resistance mutations
Virtually all patients with ALK-positive NSCLC experience progression within one to two years on first-line therapy with an ALK inhibitor due to primary or acquired resistance [5,18]. In this context, resistance may be due to ALK-specific alterations (on-target), such as ALK mutations or ALK gene amplifications, or to other mechanisms (off-target), such as activation of alternative signaling pathways [18].
Approximately 20% of patients on crizotinib and more than half on a 2nd generation ALK inhibitor (alectinib, ceritinib) develop resistance mutations [18]. The spectrum of resistance after progression under crizotinib differs markedly from that under 2nd-generation ALK inhibitors [18] . For example, the ALK p.G1202R mutation can only be detected in 2% of crizotinib-resistant biopsies, but occurs as the most common resistance mutation among 2nd generation ALK inhibitors, thereby conferring tumor resistance to all 2nd generation ALK inhibitors [18]. Each ALK inhibitor also appears to be associated with a specific spectrum of resistance mutations [18]. The 3rd generation ALK inhibitors (lorlatinib and brigatinib) cover a broader field of ALK resistance mutations (including ALK p.G1202R) than the older ALK inhibitors [18].
Second-line therapy
The choice of therapy from the second line onwards is strongly influenced by the resistance mutations present. Mutational analysis on free circulating tumor DNA (ctDNA; so-called liquid biopsy) or on a repeat tissue biopsy should therefore be used to determine the mechanism of resistance in case of progression under a 1st or 2nd generation ALK inhibitor [19]. This approach is also suggested in the ESMO guidelines as part of the decision-making process for choosing the next line of therapy [5].
Compared with chemotherapy, crizotinib significantly improved median PFS (7.7 vs. 3.0 months, HR 0.49, p<0.001) and ORR (65% vs. 20%) in the PROFILE-1007 trial in ALK inhibitor naïve patients but pre-treated with a platinum-based regimen [20]. Crizotinib is recommended as the next line of therapy if it has not been used previously [5].
In patients pretreated with chemotherapy and crizotinib, a significant improvement in median PFS of 3.8 months was observed with ceritinib compared with chemotherapy in the ASCEND-5 trial (5.4 vs. 1.6 months, HR 0.49, p<0.0001) [21]. Alectinib also significantly prolonged median PFS of 9.6 months compared with 1.4 months with chemotherapy in patients after chemotherapy and crizotinib pretreatment in the ALUR trial (HR 0.15, p<0.001) [22]. Based on these data, ceritinib or alectinib are recommended as second-line therapy after progression or intolerance with crizotinib [5].
Therapy in later lines
In later lines of therapy, the choice of available ALK inhibitors is usually limited due to existing resistance mutations. In a phase II study, the 3rd generation ALK inhibitor lorlatinib was tested in patients previously treated with two to three ALK inhibitors (1. or 2nd generation, crizotinib, alectinib, ceritinib) treated with or without chemotherapy achieved an ORR of 38.7% and a median PFS of 6.9 months [23]. In a further analysis, the efficacy of lorlatinib was investigated in the context of different ALK resistance mutations. After treatment failure with crizotinib, lorlatinib was shown to be highly effective in patients with and without ALK resistance mutations [24]. In contrast, significantly improved efficacy of lorlatinib was observed in patients who had previously received at least one ALK inhibitor in the presence of ALK resistance mutations compared with those without ALK resistance mutations [24]. ALK resistance mutations could therefore be used as biomarkers of response to lorlatinib therapy after failure under a 2nd generation ALK inhibitor [24]. Following the development of resistance to crizotinib or 2nd generation ALK inhibitors, sequential therapy with lorlatinib* or brigatinib** is the preferred approach according to ESMO guidelines [5].
Patients with brain metastases
More than 20% of patients with ALK-positive NSCLC are found to have brain metastases at initial diagnosis, and the incidence increases to more than 50% as the disease progresses [25]. One challenge in treating brain metastases is overcoming the blood-brain barrier. ALK inhibitors of the 2nd and 3rd generation showed significantly better properties in this regard compared to crizotinib [5,6].
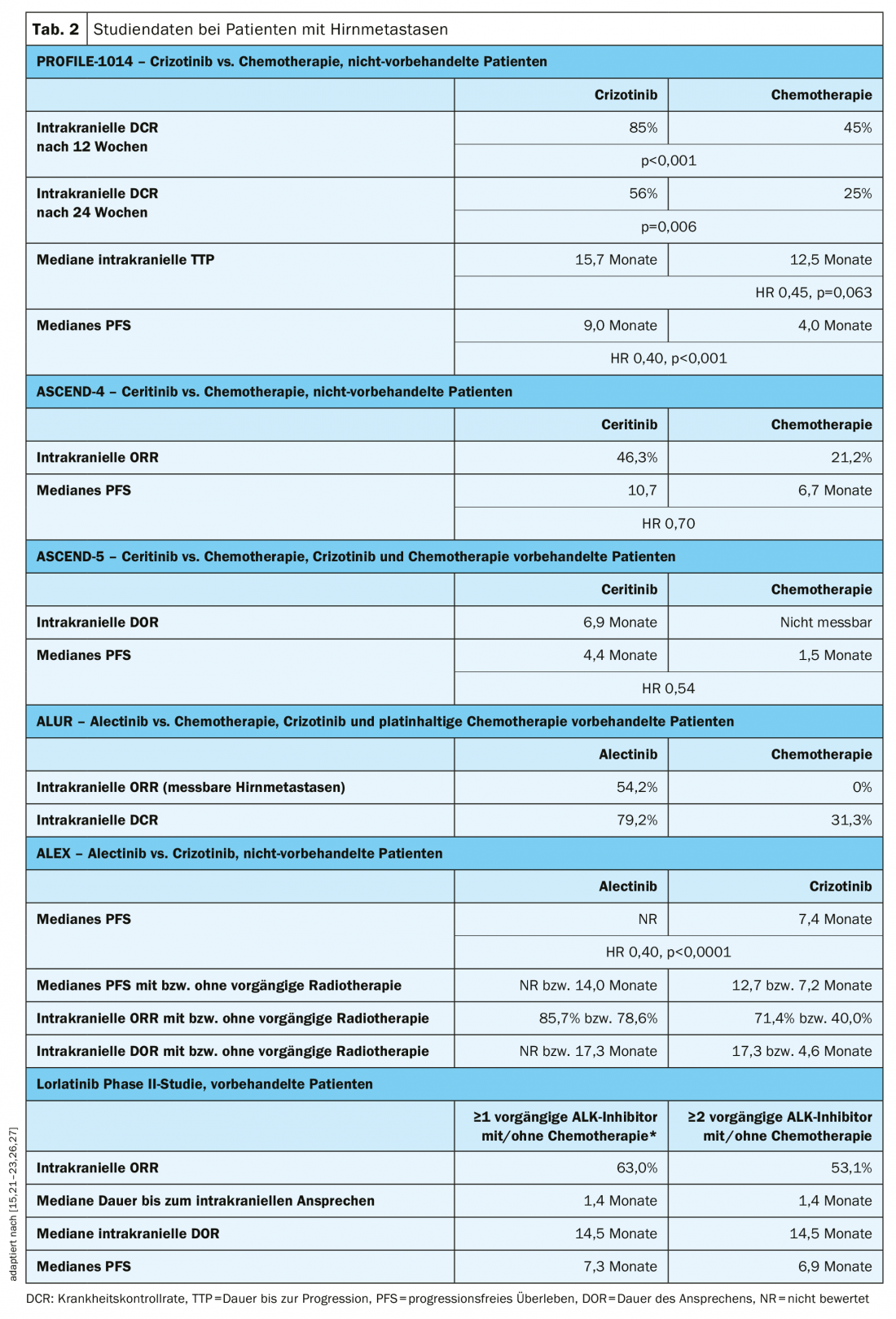
All ALK inhibitor trials included patients with brain metastases. A prospective analysis of data from patients with brain metastases in the PROFILE-1014 trial showed that significant improvement in intracranial disease control rate was achieved with crizotinib compared with chemotherapy (Table 2) [26]. The duration to intracranial tumor progression was prolonged with crizotinib, but the difference with the chemotherapy arm was not statistically significant (Table 2) [26]. Ceritinib achieved improved intracranial response and median PFS in patients with existing brain metastases compared with platinum-based chemotherapy in the first-line setting (ASCEND-4 trial) or after pretreatment with crizotinib and chemotherapy (ASCEND-5 trial), respectively (Table 2) [15,21]. In the ALUR trial, significantly higher intracranial ORR was achieved in patients with measurable brain metastases after crizotinib pretreatment with alectinib compared with chemotherapy (Table 2) [22]. Also, as first-line therapy, alectinib significantly improved median PFS in patients with brain metastases compared with crizotinib in the ALEX trial, regardless of whether radiotherapy had already been administered (Table 2) [27]. In the pivotal phase II trial, an intracranial ORR of 53.1 months and a median PFS of 6.9 months were observed with lorlatinib in patients with brain metastases after pretreatment with at least two ALK inhibitors (Table 2) [23].
Safety profiles of ALK inhibitors
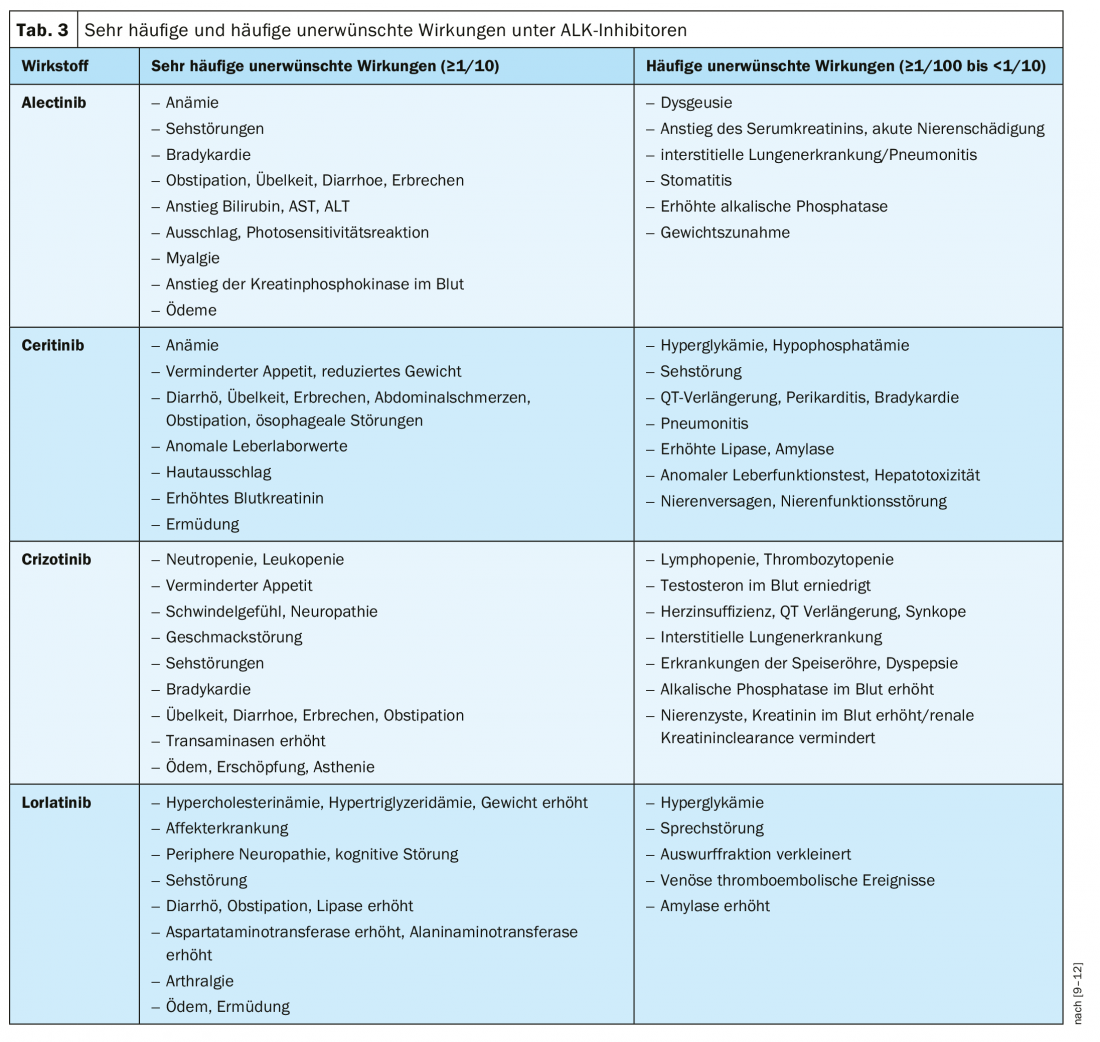
Although all ALK inhibitors can generally be considered safe, the safety profile of available agents also plays an important role in the choice of therapy, especially when in the palliative setting as in advanced ALK-positive NSCLC [28]. Table 3 summarizes the most important safety aspects of the ALK inhibitors approved in Switzerland, according to the professional information.
Sequence of ALK inhibitors
With the multitude of ALK inhibitors available, determining an optimal therapeutic sequence remains a challenge [29]. Each ALK inhibitor has unique characteristics that include central nervous system penetration, safety profile, and spectrum of activity in different resistance mutations [29].
In addition to the approval status of the various ALK inhibitors, toxicity profiles and individual patient factors play a role in the choice of first-line therapy [7]. Because crizotinib was the first ALK inhibitor available, most data are available for sequences starting with crizotinib [29]. Based on available clinical trials, crizotinib followed by alectinib or ceritinib can be expected to have a median PFS of 5.4 to 15.6 months, a median OS of 14.9 to 26.0 months, and an ORR of 33.0% to 80.0% [29]. To date, no studies are available that directly compare different ALK inhibitor sequences [29].
The ROS1-positive NSCLC
ROS1 encodes a receptor tyrosine kinase that is very similar to ALK [30]. Therefore, some of the available ALK inhibitors show high affinity for ROS1 and, accordingly, antitumor activity in patients with ROS1-positive NSCLC [31].
Crizotinib was the first ALK inhibitor also approved for the treatment of ROS1-positive NSCLC, and its efficacy in this setting has been demonstrated in several prospective and retrospective studies [30,32–39]. In the Phase I PROFILE-1001 study, an ORR of 72% was observed with crizotinib, with 11% of patients showing a complete response and 60% showing a partial response [38]. The median PFS was 19.3 months and the median OS was 51.4 months [38]. In this regard, the safety profile of crizotinib was comparable to that seen in ALK-positive NSCLC patients [38]. Crizotinib also demonstrated significant anti-tumor activity in the phase II METROS study. The median PFS was 22.8 months with an ORR of 65% [32]. ESMO guidelines recommend crizotinib monotherapy as first- or second-line therapy in patients with stage IV ROS1-positive NSCLC [5]. After first-line therapy with crizotinib, platinum-based chemotherapy can then be offered in the second line [5].
Discussion and future prospects
Treatment options in advanced ALK-positive NSCLC continue to expand. The ALK inhibitors of the 2nd generation are thereby established in the crizotinib-resistant setting and are increasingly used in the first line of therapy in patients with advanced disease [5]. With lorlatinib as an ALK inhibitor of the 3rd generation is now available as an additional option for patients after treatment failure with two ALK inhibitors [24]. In addition, further ALK inhibitors are in clinical development or close to approval.
Brigatinib is not yet approved in Switzerland, but is mentioned in the ESMO guidelines as an option in the first line of therapy. In the ALTA-1L study, a higher PFS rate was observed with brigatinib in the first line of therapy (HR 0.49, p<0.001) than with crizotinib [40]. Crizotinib-resistant patients also showed an ORR of 45% and 54% and median PFS of 9.2 and 12.9 months, respectively, in the ALTA trial of brigatinib (90 mg or 180 mg per day) [41]. The intracranial ORR was 42% and 67%, respectively, and the median intracranial PFS was 15.6 and 12.8 months, respectively [41].
In vitro, the ALK inhibitor ensartinib inhibited the growth of ALK-positive NSCLC cells 10-fold more than crizotinib [42]. In addition, in a first dose-finding study, patients treated with a dose ≥200 mg showed a response rate (RR) of 60% and a median PFS of 9.2 months. In previously ALK inhibitor naive patients, an RR of 80% and a median PFS of 26.2 months were observed [42]. Since 2016, the efficacy and safety of ensartinib compared with crizotinib has been evaluated in the open-label phase III eXalt3 trial (NCT02767804) in ALK inhibitor naïve, ALK-positive NSCLC patients [43].
In addition to various ALK inhibitor monotherapies, combination options with immune checkpoint inhibitors are also discussed as treatment options in ALK-positive NSCLC. Initial phase 1b data from the JAVELIN-101 trial showed promising antitumor activity (ORR 46.6%) and an acceptable safety profile with the anti-PD-L1 IgG1 monoclonal antibody avelumab in combination with lorlatinib [44]. The combination of alectinib with the anti-PD-L1 antibody atezolizumab also achieved initial positive results in a phase 1b study with tolerable toxicity in previously untreated patients (median follow-up 13 months, ORR 81%, median PFS 21.7 months). However, at the time of analysis, only 6 of the 21 patients were progressive [45].
Additional ALK/ROS1 inhibitors are also currently being investigated in clinical trials in ROS1-positive NSCLC. In a phase I/II study, lorlatinib achieved an ORR of 41% (95% CI 29-53) in ROS1-positive, TKI-naive, or pretreated patients (n=69) with or without CNS metastases [46]. Objective response was observed in 62% of TKI-naïve patients (10% complete response, 52% partial response), 45% of patients with CNS metastases, and 80% of the group without CNS metastases. The median time to first tumor response (TTR) was 1.4 months and the median duration of response (DOR) was 25.3 months (95% CI 7.5-31.9) [46]. The lorlatinib phase II pivotal trial enrolled a ROS1-positive cohort (EXP-6) in addition to ALK-positive patients (EXP1-5) [23,47]. A rapid and sustained response was observed with lorlatinib in this group of patients regardless of crizotinib pre-therapy [47]. The overall ORR and median PFS were 36.2% and 9.9 months, respectively, in crizotinib-naive patients 61.5% and 21.0 months, and after crizotinib pre-therapy 26.5% and 8.5 months, respectively [47]. Lorlatinib is approved in Switzerland only for the treatment of ALK-positive NSCLC [9].
Another agent being evaluated is the potent ROS1 inhibitor entrectinib as a treatment option in ROS1-positive advanced NSCLC. Integrated analysis of three phase I/II trials (ALKA-372-001, STARTRK-1, STARTRK-2) showed an ORR of 77% and median PFS of 26 and 14 months in patients without and with brain metastases, respectively [48]. Entrectinib demonstrated good tolerability and a manageable safety profile [48].
Overall, the treatment situation of patients with ALK-positive or ROS1-positive NSCLC has been greatly improved in recent years. However, further studies are needed to answer questions such as the optimal sequence of ALK inhibitors. With the expected approvals of further substances, the therapeutic options will continue to expand in the future.
Take-Home Messages
- In Switzerland, the ALK inhibitors crizotinib (1st generation), alectinib and ceritinib (2nd generation), and lorlatinib (3rd generation) are approved for the treatment of ALK-positive advanced NSCLC.
- The use of crizotinib, alectinib, or ceritinib is recommended as first-line therapy in ALK-positive NSCLC. Alectinib or ceritinib are preferred for existing brain metastases.
- Approximately 20% of patients on crizotinib and more than 50% on alectinib or ceritinib develop resistance mutations. The resistance spectrum of ALK inhibitors varies.
- After development of resistance with crizotinib, alectinib or ceritinib is recommended as the next line of therapy.
- Lorlatinib covers a broader range of resistance mutations than the ALK inhibitors of the 1. and 2nd generation and is therefore used after progression on alectinib or ceritinib.
- Crizotinib is recommended for ROS1-positive NSCLC as first- or second-line therapy.
* Lorlatinib is approved in Switzerland after progression among at least two ALK inhibitors.
** Brigatinib is not approved in Switzerland.
Literature:
- Krebsliga Schweiz – Cancer in Switzerland: important figures. As of December 2018.
- Non-Small Cell Lung Carcinoma. My Cancer Genome www.mycancer genome.org/content/disease/non-small-cell-lung-carcinoma. Last access: 10/15/2019.
- Shaw AT, et al: Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. Journal of clinical oncology: official journal of the American Society of Clinical Oncology, 2009. 27(26): 4247-4253.
- Davare MA, et al: Structural insight into selectivity and resistance profiles of ROS1 tyrosine kinase inhibitors. Proceedings of the National Academy of Sciences of the United States of America, 2015. 112(39): E5381-E5390.
- Planchard D, et al: Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol, 2019. 30(5): 863-870.
- Griesinger F, et al: Brain metastases in ALK-positive NSCLC – time to adjust current treatment algorithms. Oncotarget, 2018. 9(80): 35181-35194.
- Ziogas DC, et al: Treating ALK-positive non-small cell lung cancer. Annals of translational medicine, 2018. 6(8): 141-141.
- List of approved medicinal products for human use. www.swissmedic.ch/swissmedic/de/home/services/listen_neu.html#-257211596. Last accessed 02.03.2020.
- Current technical information Lorviqua® (lorlatinib). Status of the information: January 2020. www.swissmedicinfo.ch.
- Current SmPC Xalkori® (crizotinib). Status of the information: October 2018. www.swissmedicinfo.ch.
- Current technical information Alecensa® (alectinib). Status of the information: April 2018. www.swissmedicinfo.ch.
- Current technical information Zykadia® (ceritinib). Status of the information: May 2019. www.swissmedicinfo.ch.
- Solomon, BJ, et al: First-line crizotinib versus chemotherapy in ALK-positive lung cancer. New England Journal of Medicine, 2014. 371(23): 2167-2177.
- Solomon BJ, et al: Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol, 2018. 36(22): 2251-2258.
- Soria JC, et al: First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet, 2017. 389(10072): 917-929.
- Peters S, et al: Alectinib versus crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. New England Journal of Medicine, 2017. 377(9): 829-838.
- Hida T, et al: Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet, 2017. 390(10089): 29-39.
- Gainor JF, et al: Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer discovery, 2016. 6(10): 1118-1133.
- McCusker MG, et al: How I treat ALK-positive non-small cell lung cancer. ESMO Open, 2019. 4(Suppl 2): e000524.
- Shaw AT, et al: Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med, 2013. 368(25): 2385-2394.
- Shaw, A.T., et al: Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol, 2017. 18(7): 874-886.
- Novello S, et al: Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Annals of oncology : official journal of the European Society for Medical Oncology, 2018. 29(6): 1409-1416.
- Solomon BJ, et al: Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol, 2018.
- Shaw AT, et al: ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. Journal of Clinical Oncology, 2019. 37(16): 1370-1379.
- Rangachari D, et al: Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung cancer (Amsterdam, Netherlands), 2015. 88(1): 108-111.
- Solomon BJ, et al: Intracranial Efficacy of Crizotinib Versus Chemotherapy in Patients With Advanced ALK-Positive Non-Small-Cell Lung Cancer: Results From PROFILE 1014. J Clin Oncol, 2016. 34(24): 2858-2865.
- Gadgeel S, et al: Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Annals of oncology: official journal of the European Society for Medical Oncology, 2018. 29(11): 2214-2222.
- Zhu Q, et al: Pooled safety analyses of ALK-TKI inhibitor in ALK-positive NSCLC. BMC cancer, 2017. 17(1): 412-412.
- Barrows SM, et al: Systematic review of sequencing of ALK inhibitors in ALK-positive non-small-cell lung cancer. Lung Cancer (Auckland, N.Z.), 2019. 10: 11-20.
- Michels S, et al: Safety and Efficacy of Crizotinib in Patients With Advanced or Metastatic ROS1-Rearranged Lung Cancer (EUCROSS): A European Phase II Clinical Trial. Journal of Thoracic Oncology, 2019. 14(7): 1266-1276.
- Sehgal K, et al: Targeting ROS1 rearrangements in non-small cell lung cancer with crizotinib and other kinase inhibitors. Translational cancer research, 2018. 7(Suppl 7): S779-S786.
- Landi L, et al: Crizotinib in MET deregulated or ROS1 rearranged pretreated non-small-cell lung cancer (METROS): a phase II, prospective, multicentre, two-arm trial. Clinical Cancer Research, 2019.
- Liu C, et al: Crizotinib in Chinese Patients with ROS1-Rearranged Advanced Non-Small-Cell Lung Cancer in Routine Clinical Practice. Targeted oncology, 2019: 1-9.
- Masuda K, et al: Efficacy and safety of crizotinib in patients with ROS1 rearranged non-small cell lung cancer: a retrospective analysis. Journal of thoracic disease, 2019. 11(7): 2965.
- Mazières J, et al: Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: results from the EUROS1 cohort. Journal of clinical oncology, 2015. 33(9): 992-999.
- Moro-Sibilot D, et al: Crizotinib in c-MET- or ROS1-positive NSCLC: results of the AcSé phase II trial. Annals of Oncology, 2019.
- Park S, et al: Characteristics and outcome of ROS1-positive non-small cell lung cancer patients in routine clinical practice. Journal of Thoracic Oncology, 2018. 13(9): 1373-1382.
- Shaw A, et al: Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Annals of Oncology, 2019. 30(7): 1121-1126.
- Wu YL., et al: Phase II study of crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. Journal of Clinical Oncology, 2018. 36(14): 1405-1411.
- Camidge DR, et al: Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. New England Journal of Medicine, 2018. 379(21): 2027-2039.
- Kim DW, et al: Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol, 2017. 35(22): 2490-2498.
- Horn L, et al: Ensartinib (X-396) in ALK-Positive Non-Small Cell Lung Cancer: Results from a First-in-Human Phase I/II, Multicenter Study. Clinical cancer research: an official journal of the American Association for Cancer Research, 2018. 24(12): 2771-2779.
- Horn L, et al: eXalt3: A phase III study of ensartinib (X-396) in anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC). Journal of Clinical Oncology, 2017. 35(15_suppl): TPS8578-TPS8578.
- Shaw AT, et al: Avelumab (anti-PD-L1) in combination with crizotinib or lorlatinib in patients with previously treated advanced NSCLC: phase 1b results from JAVELIN Lung 101. Journal of Clinical Oncology, 2018. 36(15_suppl): 9008-9008.
- Kim DW, et al: Safety and clinical activity results from a phase Ib study of alectinib plus atezolizumab in ALK+ advanced NSCLC (aNSCLC). Journal of Clinical Oncology, 2018. 36(15_suppl): 9009-9009.
- Shaw AT, et al: Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol, 2019. 20(12): 1691-1701.
- Ou S, et al: OA02.03 Clinical Activity of Lorlatinib in Patients with ROS1 Advanced Non-Small Cell Lung Cancer: Phase 2 Study Cohort EXP-6. Journal of Thoracic Oncology, 2018. 13(10): S322-S323.
- Barlesi F, et al: Entrectinib in locally advanced or metastatic ROS1 fusion-positive non-small cell lung cancer (NSCLC): integrated analysis of ALKA-372-001, STARTRK-1 and STARTRK-2. Ann Oncol, 2019. 30(suppl_2):109O.
InFo ONCOLOGY & HEMATOLOGY 2020; 8(2): 14-20 (published 4/23/20, ahead of print).