Psoriasis is nowadays understood as a systemic disease, which is based on chronic inflammation. Research into the etiopathogenesis, as well as the establishment of new active substances, is progressing rapidly. A promising new approach is TYK2 inhibition. In pivotal clinical trials, the first oral selective tyrosine kinase 2 inhibitor in psoriasis showed promising results.
For patients with moderate to severe psoriasis, there are many safe and effective biologic therapies available in addition to conventional systemic therapies. However, some patients prefer oral therapeutics [1]. TYK2 inhibition using deucravacitinib (BMS-986165) may fill this therapeutic gap.
Deucravacitinib is a highly selective oral tyrosine kinase 2 inhibitor
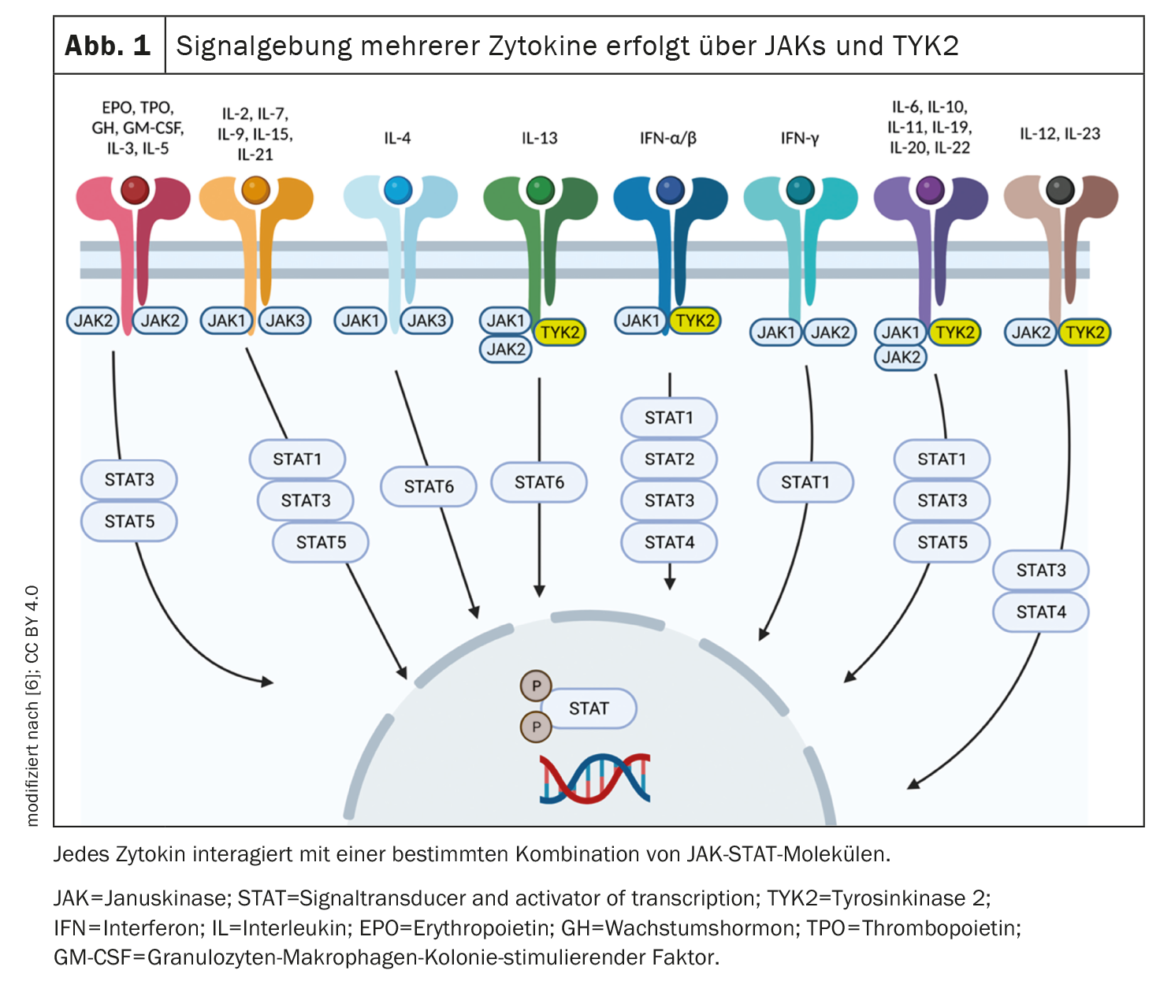
In contrast to inhibitors of JAK1-3, deucravacitinib selectively blocks IL-23, IL-12, and type I IFN signaling by binding to the regulatory domain, but not those of IL-6, hematopoietic growth factors, and the γc-receptor family (Fig. 1) [2]. Thus, deucravacitinib achieves highly selective effects, which likely contributes to an improved safety profile.
In a randomized-controlled double-blind phase II dose-finding study including 267 patients with moderate-to-severe psoriasis, deucravacitinib showed clinical efficacy at a daily dose of 3 mg and higher (NCT02931838) [3]. The proportion of patients with a 75% or greater reduction in PASI at week 12 was significantly higher in patients receiving deucravacitinib than in the placebo group (at 3 mg daily or twice daily, at 6 mg twice daily, and at 12 mg daily) (p<0.001) [3].
Phase III study program POETYK: promising results
In the EU, a recommendation of the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency is currently under review [4]. The CHMP based its positive opinion on the results of the pivotal Phase III POETYK PSO-1 and POETYK PSO-2 studies, which evaluated once-daily administration of deucravacitinib in patients with moderate-to-severe plaque psoriasis compared to placebo and twice-daily apremilast, as well as additional two-year data from the long-term extension study POETYK PSO. Results from the 52-week, double-blind, phase III POETYK PSO-1 trial (NCT03624127) were published in The Journal of the American Academy of Dermatology 2023 [5]. Participants were randomized 2:1:1 to receive either deucravacitinib 6 mg daily (n=332), placebo (n=166), or apremilast 30 mg twice daily (n=168). At week 16, response rates were significantly higher with deucravacitinib than with placebo or apremilast for both PASI 75** (58.4% vs. 12.7% vs. 35.1%; p <0.0001) and sPGA£ 0/1 (53.6% vs. 7.2% vs. 32.1%; p<0.0001). Efficacy was maintained until week 52. The rate of adverse events with deucravacitinib was comparable to the placebo and apremilast study arms.
** PASI75=at least 75% reduction in Psoriasis Area and Severity Index.
£ sPGA 0/1=static Physician’s Global Assessment Score of 0 or 1.
Literature:
- Feldman SR, et al: Relative importance of mode of administration in treatment preferences among plaque psoriasis patients in the United States. JHEOR 2016; 4(2): 141-157.
- Ghoreschi K, et al: TYK2 inhibition: potential in the treatment of chronic inflammatory immune diseases. JDDG 2021; 19(10): 1409-1420.
- Papp K, et al: Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med 2018; 379(14): 1313-1321.
- European Medicines Agency (EMA): Summary of
Opinion, 01/26/2013, www.ema.europa.eu/en/documents/smop-initial/chmp-summary-positive-opinion-sotyktu_en.pdf,(last accessed 02/09/2023). - Armstrong AW, et al: Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. JAAD 2023; 88(1): 29-39.
- Rusiñol L, Puig L: Tyk2 Targeting in Immune-Mediated Inflammatory Diseases. International Journal of Molecular Sciences 2023; 24(4): 3391.
https://doi.org/10.3390/ijms24043391.
DERMATOLOGIE PRAXIS 2023; 33(1): 28











