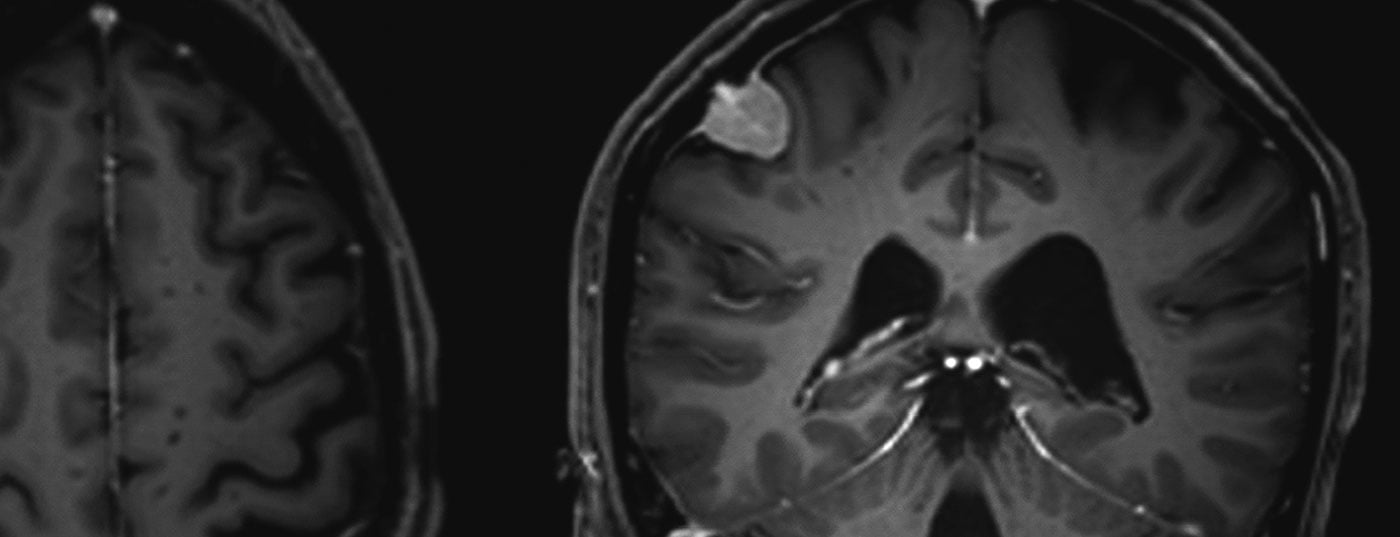
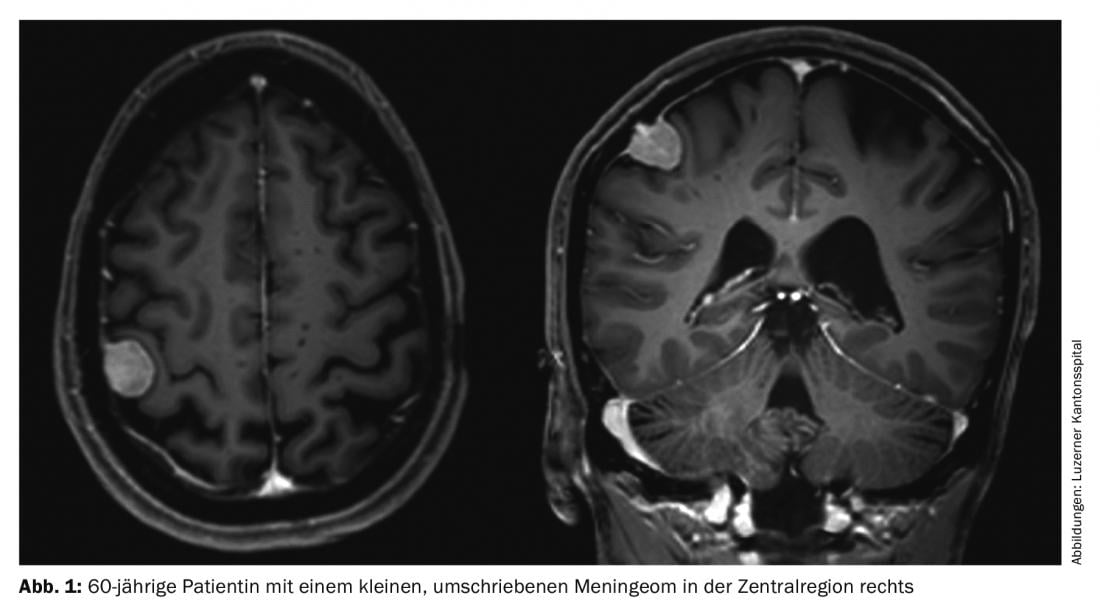
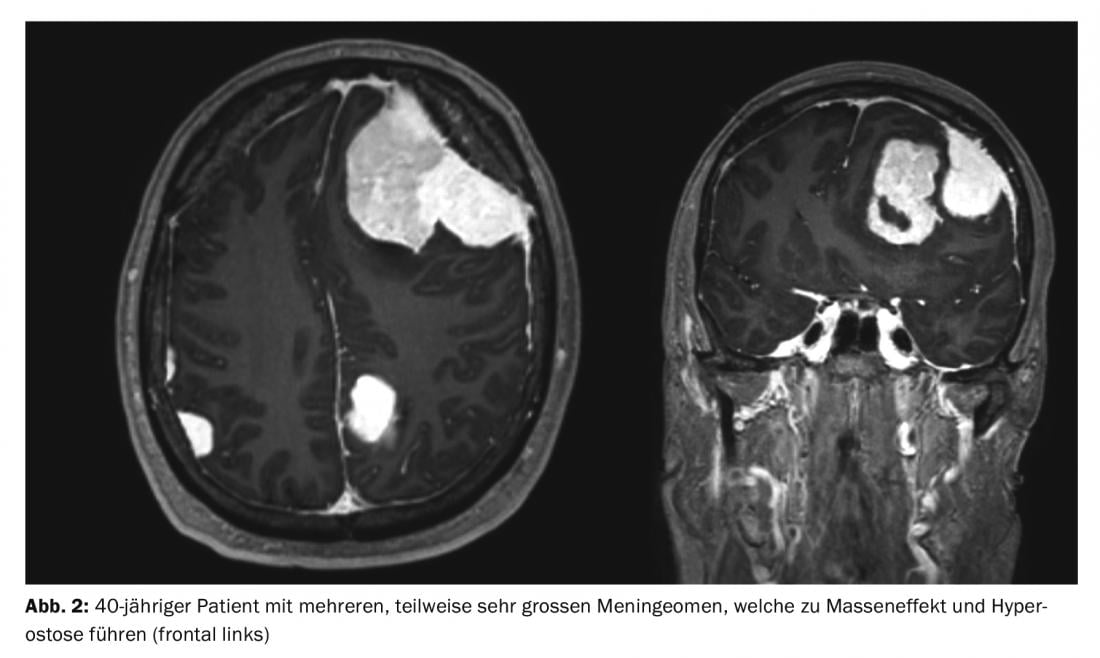
Surgery is the main form of therapy for meningioma, followed by radiotherapy. The latter is used for higher grade tumors, those with non-removable remnants, or for certain meningiomas in the base of the skull.
Meningiomas account for about one-third of all primary tumors of the central nervous system. An incidence of 7.61/100,000 population is reported from the United States in 2008 to 2012 [1]. These are predominantly benign and slow-growing tumors. These can affect the entire neural axis. They arise from the arachnoid meningothelial cells and are therefore mostly located intracranially-extraaxially (cranial) or intradurally-extramedullary (spinal). Multiple tumors are present in 8% of cases (Figs. 1 and 2) [2], especially in genetic syndromes such as neurofibromatosis. Spinal localization is found in approximately 10% of cases. Intracranial meningiomas are most commonly located along the falx cerebri, sphenoid wing, or over the convexity [3].
Histology and WHO classification 2016
Meningiomas are classified according to the WHO classification of brain tumors into grades I (benign), II (atypical), and III (anaplastic meningiomas that can metastasize systemically and have high recurrence rates after resection) [4].
The diagnosis of meningioma is usually made histologically on sections stained with hematoxylin and eosin based on typical criteria such as whorl formation, nuclear pseudoinclusions, or evidence of psammoma bodies (concentrically layered calcifications) [4,5]. Histological diagnosis can be confirmed with immunohistochemistry, e.g. for the markers epithelial membrane antigen (EMA) or somatostatin receptor 2a.
Since the new WHO classification of 2016, invasion of brain tissue, which is not rare in itself, is now considered a criterion for WHO grade II (atypical meningioma), even if no other atypia criteria are present [4]. This is based on the comparable recurrence and mortality rates of meningiomas with brain parenchymal invasion and those containing histologic atypia signs [6]. Furthermore, these atypia criteria apply (at least three of the following five histologic criteria met):
- Necrosis
- Sheeting (loss of the vortex formation or the
- fascicular histoarchitecture).
- Prominent nucleoli
- High cellularity
- Small cells [4,6].
Anaplastic meningiomas WHO grade III, about 2% of all meningiomas, show clustered mitoses (>20 mitoses in ten visual fields) and invasion of brain tissue, often relapse early even after total resection, and very rarely may metastasize outside the central nervous system [7].
Molecular markers
Molecular diagnostics is also playing an increasingly important role in meningiomas. For example, there is preliminary evidence that telomerase reverse transcriptase (TERT) mutations lead to more aggressive tumor growth in meningiomas, independent of individual WHO grade [8,9].
Most meningiomas have only one copy from the long arm of chromosome 22 (22q) [10]. Neurofibromin 2, a gene localized to 22q, is also often additionally mutated; this is also the case in neurofibromatosis 2, which is characterized, among other things, by multiple meningiomas [11]. Certain chromosomal aberrations, such as loss from the short arm of chromosome 1 (1p), the long arm of chromosome 10 (10q) or 14 (14q), are associated with increased risk of malignancy and recurrence [12,13]. Intensive research is being conducted with the aim of integrating the molecular markers, similar to other brain tumors, into the WHO classification and thus enabling more targeted diagnostics and possibly, in the future, more individualized therapy in the sense of so-called “targeted therapies”.
Clinic
The clinic depends on the location and size of the meningiomas. Supratentorial meningiomas may present with neurologic deficits of any type or epileptic seizures. Patients are adjusted with antiepileptic drugs in case of symptomatic epilepsy, which can then also be phased out after complete removal of the meningiomas in the course of several weeks postoperatively after normalization of the electroencephalography. Spinal meningiomas typically result in nocturnal pain that is usually diffuse but may also be girdled radicular at the level of the tumor. An insidious, slowly progressive paraparesis, initially manifesting simply as gait disturbance, is not infrequently recognized late because it usually involves elderly patients where other forms of gait disturbance are common. Because the clinic is usually caused by local compression rather than invasion by the tumor itself, the prognosis for recovery from neurologic deficits is favorable with prompt surgery.
With the increasing use of MRI in clinical practice, meningiomas are being detected more frequently by chance (in up to 1% of all MRIs).
Imaging
Usually, contrast-enhanced magnetic resonance imaging is used for the diagnosis and follow-up of meningiomas; in cases of contraindications, contrast-enhanced computed tomography is used alternatively [14,15].
Perfusion magnetic resonance imaging can be used to differentiate meningiomas from other entities, as meningiomas usually have increased relative cerebral blood volume (rCBV) and other lesions (e.g., dural metastases) do not [16]. Using peptide ligands such as 90Y-dotatoc or 68Ga-dotatate as tracers for positron emission tomography (PET), somatostatin receptor 2 expression can be used to differentiate meningiomas from healthy tissue [17,18]. On magnetic resonance spectroscopy, meningiomas show a characteristic alanine peak between 1.3 and 1.5 parts per million (“ppm”) [19].
Management and therapy
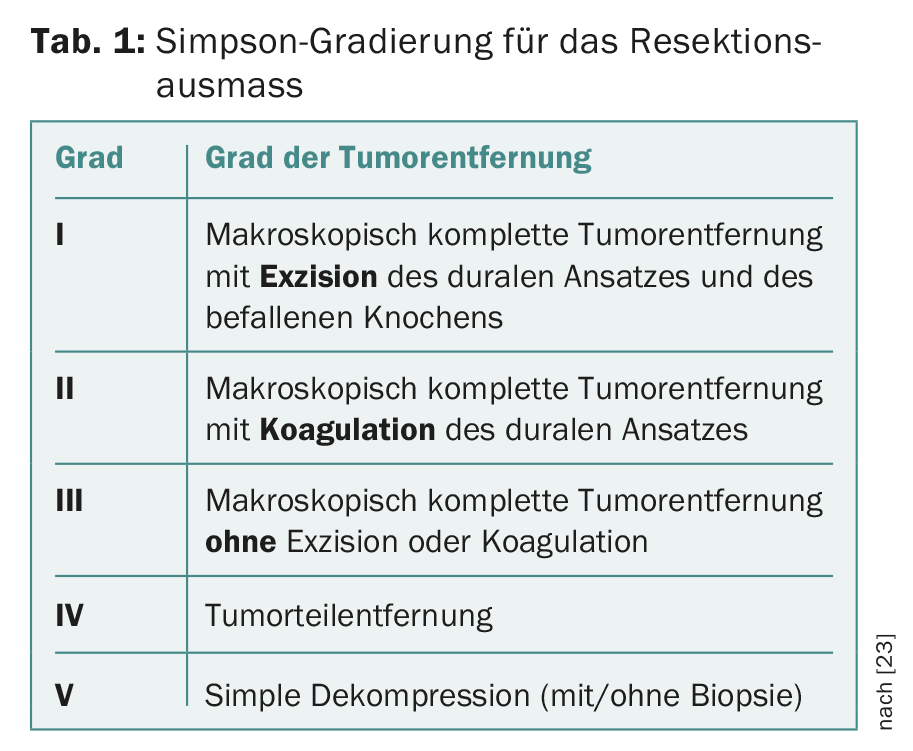
Nowadays, meningiomas are often discovered by chance: an MRI to clarify headaches rarely reveals a cause of pain, but not infrequently reveals a chance finding. For further management of such asymptomatic lesions with clinical and imaging follow-up at first semiannual and then annual intervals, there is little and relatively weak evidence in the literature [14,20–22]. If therapy becomes necessary due to clinical symptoms or growth documented by imaging, microneurosurgical resection is virtually always the first-line therapy [14]. The goal of surgery should be – whenever possible – a complete resection of the tumor including affected dura and affected bone. The resection extent of meningiomas and, accordingly, their risk of recurrence is still classified according to the Simpson classification of 1957 [23] (Table 1) [22,24].
Preoperative embolization of meningiomas can simplify surgery in individual cases, e.g., when the blood supply to the tumor cannot be controlled well by surgical means or, for anatomic reasons, only at the end of resection. WHO grade III, anaplastic meningiomas must be surgically operated on as radically as possible to reduce the risk of recurrence and metastasis as much as possible [14].
Radiosurgery (stereotactic single-dose convergent irradiation) can be used for small tumors, for those that cannot be operated on or can only be operated on incompletely, for localization in the region of the cavernous sinus or the petrous apex, or for elderly patients and high comorbidity. In certain cases, e.g. in larger tumors in the area of the petrous apex or the cavernous sinus, the radiation dose can also be applied as fractionated radiotherapy divided into several doses. In WHO grade II meningiomas, patient recruitment is currently underway for the ROAM/EORTC 1308 trial (ISRCTN71502099), in which patients with tumor resections corresponding to Simpson grade I-III are randomly assigned to early postoperative radiotherapy or placebo therapy to investigate whether radiation can reduce the risk of tumor recurrence or prolong the recurrence-free time [25]. Currently, adjuvant fractionated radiotherapy is recommended for incompletely resected WHO grade II meningiomas or for WHO grade II tumors with progression [26–28], although individual studies have shown unclear results or no convincing benefit [29–32]. Similarly, fractionated radiotherapy is recommended postoperatively for WHO grade III meningiomas, regardless of the extent of resection [14].
Pharmacotherapy plays a completely minor role in the treatment of meningiomas. This may change in the future with the discovery of potential targets for “targeted therapies”.
Operation
A meningioma is removed using microsurgical techniques. When localized near eloquent structures such as the brainstem or spinal cord, surgery is performed with intraoperative neurophysiologic monitoring, primarily motor evoked potentials.
For intracranial tumors, neuronavigation technology is used for surgical planning to achieve the most tumor-centered approach possible. This should be as small and gentle as possible, but still large enough to provide sufficient visibility of the tumor margins. Spinal height localization is simply done with X-ray or fluoroscopy. After laminotomy or hemilaminectomy and before dura opening, correct exposure is checked with ultrasound. For access, the laminae of the affected vertebral bodies are reinserted and refixed with mini titanium plates (laminotomy/laminoplasty). Increasingly, minimally invasive approaches such as hemilaminectomies are being used, which require only unilateral exposure and allow for more rapid recovery with less pain.
Meningiomas are removed as completely as possible after craniotomy is performed, preferably in one piece and with their site of attachment to the dura mater. Affected bone is also removed if technically possible. The dura is reconstructed with autologous (periosteum from the cranium) or foreign materials (dura replacement patches made of synthetic or xenogenic materials). Such a resection with complete tumor removal including excision of the dural attachment and excision of the affected bone is classified according to Simpson grade I (Tab. 1) . In cases where this is technically not possible, e.g. near the base of the skull, a less destructive resection (Simpson grade II-III) is performed. Biopsies (Simpson V) for meningiomas are an extremely rare exception.
Postoperatively, patients are monitored in the intensive care unit for one night as standard. On the first postoperative day, an MRI is also performed as a standard procedure to document residual tumor and any complications (ischemia, hemorrhage, hydrocephalus). Unexpectedly much residual tumor, which could lead to early reoperation, almost never occurs in meningioma surgery. The median length of hospital stay is four to five days in uncomplicated cases, and convalescence is about six weeks.
Aftercare
Postoperative follow-up is performed by serial MR examinations. For WHO grade I meningiomas, an annual interval is recommended for the first five years and a biennial clinical and imaging follow-up interval thereafter [14]. For WHO grade II meningiomas, a shorter follow-up interval of six months is recommended [33], and for WHO grade III meningiomas, even a three- to six-month follow-up interval is recommended [14].
Forecast
The 5-year survival rate of all meningiomas combined is about 90% and depends mainly on the recurrence rate (WHO grade I: 10%/WHO grade II: 30%/WHO grade III: 50%) [34].
Resection extent of tumors by Simpson grading (Table 1) isassociated with prognosis, especially for convexity meningiomas; grading seems to be less relevant for falx or skull base meningiomas [35]. An overall association seems to be more with recurrence-free survival and less with overall survival [36].
Take-Home Messages
- Until further notice, surgical therapy is the first and most important form of therapy, followed by radiotherapy.
- Radiotherapy is used for higher grade tumors, those with non-removable remnants, or for certain meningiomas in the skull base.
- Meningiomas with brain invasion are considered grade II tumors according to the new 2016 WHO classification.
Literature:
- Ostrom QT, et al: CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol 2015; 17(Suppl 4): iv1-iv62.
- Sheehy JP, Crockard HA: Multiple meningiomas: a long-term review. J Neurosurg 1983; 59(1): 1-5.
- Yamashita J, et al: Recurrence of intracranial meningiomas, with special reference to radiotherapy. Surg Neurol 1980; 14(1): 33-40.
- Louis DN, et al: The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016; 131(6): 803-820.
- Nowosielski M, et al: Diagnostic Challenges in Meningioma. Neuro Oncol 2017. DOI: 10.1093/neuonc/nox101 [Epub ahead of print].
- Perry A, et al: Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol 1997; 21(12): 1455-1465.
- Thomas HG, Dolman CL, Berry K: Malignant meningioma: clinical and pathological features. J Neurosurg 1981; 55(6): 929-934.
- Sahm F, et al: AKT1E17K mutations cluster with meningothelial and transitional meningiomas and can be detected by SFRP1 immunohistochemistry. Acta Neuropathol 2013; 126(5): 757-762.
- Goutagny S, et al: High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol 2014; 24(2): 184-189.
- Dumanski JP, et al: Deletion mapping of a locus on human chromosome 22 involved in the oncogenesis of meningioma. Proc Natl Acad Sci U S A 1987; 84(24): 9275-9279.
- Rouleau GA, et al: Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 1993; 363(6429): 515-521.
- Tabernero MD, et al: Characterization of chromosome 14 abnormalities by interphase in situ hybridization and comparative genomic hybridization in 124 meningiomas: correlation with clinical, histopathologic, and prognostic features. Am J Clin Pathol 2005; 123(5): 744-751.
- Maillo A, et al: Early recurrences in histologically benign/grade I meningiomas are associated with large tumors and coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone. Neuro Oncol 2007; 9(4): 438-446.
- Goldbrunner R, et al: EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol 2016; 17(9): e383-391.
- Saloner D, et al: Modern meningioma imaging techniques. J Neurooncol 2010; 99(3): 333-340.
- Kremer S, et al: Contribution of dynamic contrast MR imaging to the differentiation between dural metastasis and meningioma. Neuroradiology 2004; 46(8): 642-648.
- Collamati F, et al: Toward radioguided surgery with β- decays: uptake of a somatostatin analogue, DOTATOC, in meningioma and high-grade glioma. J Nucl Med 2015; 56(1): 3-8.
- Rachinger W, et al: Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med 2015; 56(3): 347-353.
- Majós C, et al: Intraventricular meningiomas: MR imaging and MR spectroscopic findings in two cases. AJNR Am J Neuroradiol 1999; 20(5): 882-885.
- Vernooij MW, et al: Incidental findings on brain MRI in the general population. N Engl J Med 2007; 357(18): 1821-1828.
- Sughrue ME, et al: Treatment decision making based on the published natural history and growth rate of small meningiomas. J Neurosurg 2010; 113(5): 1036-1042.
- Sughrue ME, et al: The relevance of Simpson Grade I and II resection in modern neurosurgical treatment of World Health Organization Grade I meningiomas. J Neurosurg 2010; 113(5): 1029-1035.
- Simpson D: The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 1957; 20(1): 22-39.
- Gousias K, Schramm J, Simon M: The Simpson grading revisited: aggressive surgery and its place in modern meningioma management. J Neurosurg 2016; 125(3): 551-560.
- Jenkinson MD, et al: The ROAM/EORTC-1308 trial: radiation versus observation following surgical resection of Atypical Meningioma: study protocol for a randomised controlled trial. Trials 2015; 16: 519.
- Aghi MK, et al: Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery 2009; 64(1): 56-60; discussion 60.
- Komotar RJ, et al: The role of radiotherapy following gross-total resection of atypical meningiomas. J Neurosurg 2012; 117(4): 679-686.
- Park HJ, et al: The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol 2013; 115(2): 241-247.
- Hammouche S, et al: Long-term survival analysis of atypical meningiomas: survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir (Vienna) 2014; 156(8): 1475-1481.
- Mair R, et al: Radiotherapy for atypical meningiomas. J Neurosurg 2011; 115(4): 811-819.
- Stessin AM, et al: Does adjuvant external-beam radiotherapy improve outcomes for nonbenign meningiomas? A Surveillance, Epidemiology, and End Results (SEER)-based analysis. J Neurosurg 2012; 117(4): 669-675.
- Yoon H, et al: Atypical meningioma: randomized trials are required to resolve contradictory retrospective results regarding the role of adjuvant radiotherapy. J Cancer Res Ther 2015; 11(1): 59-66.
- Marosi C, et al: Meningioma. Crit Rev Oncol Hematol 2008; 67(2): 153-171.
- Mahaley MS, et al: National survey of patterns of care for brain-tumor patients. J Neurosurg 1989; 71(6): 826-836.
- Voss KM, et al: The Simpson grading in meningioma surgery: does the tumor location influence the prognostic value? J Neurooncol 2017; 133(3): 641-651.
- Nanda A, et al: Relevance of Simpson grading system and recurrence-free survival after surgery for World Health Organization grade I meningioma. J Neurosurg 2017; 126(1): 201-211.
InFo ONCOLOGY & HEMATOLOGY 2017; 5(6): 11-15.