In recent years, the therapeutic landscape for type 2 diabetes has changed significantly. Because GLP-1 receptor agonists and SGLT-2 inhibitors have shown direct effects on cardiorenal protection in cardiovascular endpoint studies independent of glycemic control, these classes of agents are now recommended as first-line therapy by the Swiss Society of Endocrinology and Diabetology (SGED).
About 90% of all adult diabetics suffer from type 2 diabetes (T2D), i.e., insulin resistance with relative insulin deficiency [1]. According to international diabetes societies, T2D is said to occur when the following criteria are met in individuals with diabetes-typical symptoms or in the presence of an increased risk of diabetes [2,3]:
- Occasional plasma glucose level ≥11.1 mmol/l (≥200 mg/dl)
- Fasting plasma glucose ≥7.0 mmol/l (≥126 mg/dl)
- 2-h plasma glucose level on an oGTT (oral glucose tolerance test) ≥11.1 mmol/l (≥200 mg/dl).
- HbA1c value ≥48 mmol/mol (≥6.5%)
The Swiss Society of Endocrinology and Diabetology regularly develops updated recommendations for diabetes management. The most recent version of the treatment recommendations for type 2 diabetes appeared in 2023 in the journal Swiss Medical Weekly under the title “Swiss recommendations of the Society for Endocrinology and Diabetes (SGED/SSED) for the treatment of type 2 diabetes mellitus” [4]. The following is a compact summary of some of the important points addressed therein.
Figure 1 provides an overview outline of the recommended treatment algorithm.
Combine SGLT-2-i or GLP-1-RA with metformin from the start.
Nowadays, a multifactorial approach to diabetes management is advocated, in which lifestyle measures also play an important role (box). For the initial drug treatment of type 2 diabetes, a combination of metformin and an SGLT-2 inhibitor (SGLT-2-i) or a GLP-1 receptor agonist (GLP-1-RA) has recently been advocated for all patients right from the start. Metformin is maintained as a first-line baseline therapeutic because the large cardiovascular endpoint studies have been based on metformin treatment and because no other antidiabetic currently reduces hepatic glucose production as markedly [5–13].
| Multifactorial treatment approach As a first step in the treatment of (pre)diabetes, the authors recommend lifestyle changes for all age groups. Healthy eating, weight control and physical activity play a key role and should ideally be implemented in parallel. However, a multifactorial approach also includes treatment of arterial hypertension, reduction of LDL cholesterol, and smoking cessation. The importance of multifactorial treatment (box) in the therapy of type 2 diabetes was demonstrated, among others, in the Steno-2 study. |
| to [4,29] |
If this initial dual treatment regimen is not sufficient to achieve the target HbA1c, it is suggested that either a GLP-1 RA or an SGLT-2 inhibitor be added as a third medication. This triple combination has not been formally studied in cardiovascular endpoint studies. But a growing body of clinical experience in Europe and the United States demonstrates that, compared with other combinations, it is the best option for reducing 3-point MACE (“major adverse cardiac event”), all-cause mortality, and heart failure [14,15].
| Set HbA1c target values individually The primary goal of diabetes control is to keep HbA1c within the normal range as much as possible and to avoid hypoglycemia. In most patients, the HbA1c target value is around 7.0%. In younger individuals with a short history of diabetes or microvascular complications, the target value should be reduced to 6.5% if this can be achieved without significant and repeated hypoglycemia. Unless insulin or sulfonylurea is used, an HbA1c level <6.5% is not dangerous in terms of hypoglycemia or cardiovascular complications. For elderly patients, those with a history of severe hypoglycemia, or if comorbidities are present (e.g., visual impairment, osteoporosis, neurologic disease** or if life expectancy is limited, it may be appropriate to aim for an HbA1c target of 7-8%. However, an HbA1c level >8.0% should be avoided in all cases because the associated complications outweigh the potential benefit of a higher HbA1c. |
| ** e.g. autonomic neuropathy |
| according to [4] |
Weight control – GLP-1-RA and GIP-RA score points
The updated SGED guideline discusses weight management in detail. 60-90% of all people with type 2 diabetes are obese. Therefore, in addition to preventing microvascular and macrovascular complications, a major goal of diabetes management is to reduce body weight [16]. In contrast to SGLT-2 inhibitors, GLP-1 receptor agonists produce more substantial weight loss and should therefore be used in preference to SGLT-2 inhibitors in obese patients with type 2 diabetes [5–13]. Weight loss increases during dose escalation. Specifically, it has been shown that semaglutide at high doses (2.4 mg) is currently the GLP-1 RA that is most effective in reducing weight. This is the second GLP-1 RA, after high-dose liraglutide (3.0 mg), approved for treatments aimed at weight loss. Tirzepatide, a dual GLP-1 and GIP receptor agonist with highly effective glycemic control and weight-loss effects compared with semaglutide, is nearing market approval. For BMI >28 kg/m², the use of GLP-1 receptor agonists in T2D therapy – in combination with metformin or as monotherapy if metformin is not tolerated – is reimbursed by health insurers.
T2D with impaired renal function – what to consider?
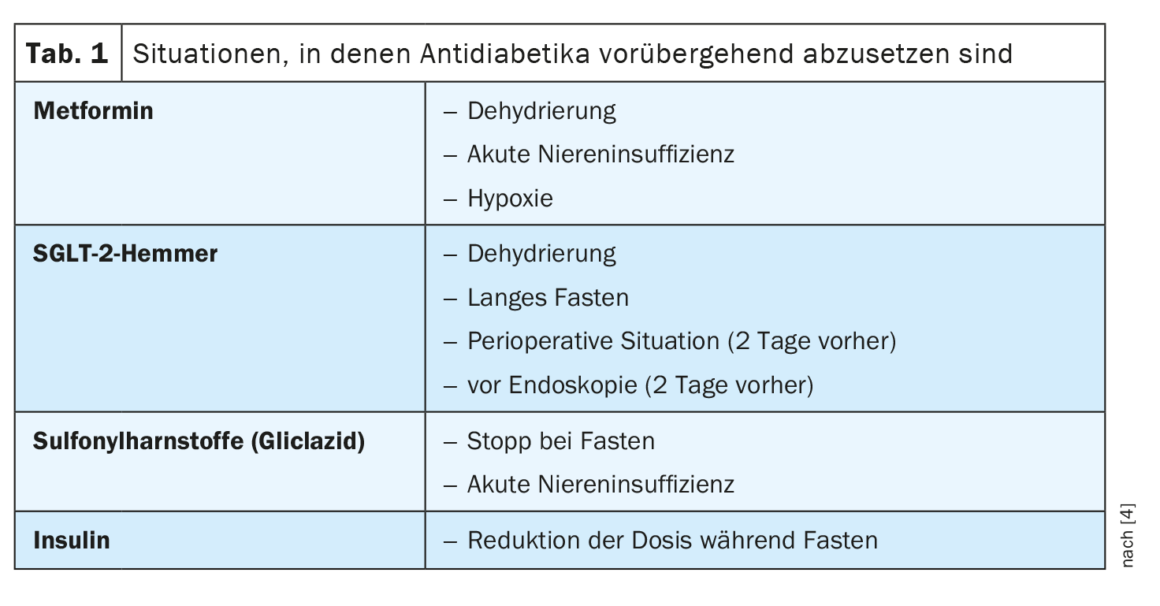
SGLT-2-i have shown exceedingly beneficial cardiorenal effects in both diabetic and non-diabetic patients [17]. Thus, in patients with chronic kidney disease (CKD), SGLT-2-i reduced not only renal and cardiovascular endpoints but also mortality [17]. In the presence of markedly reduced GFR, the blood glucose-lowering efficacy of SGLT-2-i is reduced or absent, but the nephroprotective effects are maintained. However, use of SGLT-2-i tends to be discouraged in GFR <30 ml/min because of limited experience with this patient subpopulation. Metformin must be discontinued if GFR is less than 30 ml/min due to risk of lactic acidosis. With a GFR of 30-45 ml/min, the maximum daily dose of metformin is 2× 500 mg or 1000 mg in sustained-release form.
GLP-1-RA also have nephroprotective effects, but not to the same extent as SGLT-2-i. GLP-1-RA can be used in patients (with BMI >28 kg/m²) without dose adjustment, even if their GFR is severely reduced or they are undergoing dialysis. DPP-4 inhibitors do not exhibit nephroprotective effects in the short term but can be given as an alternative to GLP-1-RA (e.g., in patients who have a BMI<28 kg/m² or cannot tolerate GLP-1-RA) [18–21]. DPP-4 inhibitors are safe to use in patients with reduced GFR, but the dose must be adjusted for renal function (except for linagliptin). Sulfonylureas, including gliclazide, should not be used in patients with eGFR <30 ml/min because of the increased risk of hypoglycemia.
Heart failure – SGLT-2-i with additional benefit
Up to 30% of diabetics over 65 years of age have heart failure ( HF)£, even independent of the presence of other cardiovascular risk factors [22,23]. At the latest when the suspected diagnosis of heart failure is confirmed by transthoracic echocardiography, the use of an SGLT-2-i is reasonable, because agents of this substance class have proven to be effective for the prevention or treatment of HFrEF as well as HFpEF and HFmEF, regardless of whether diabetes is present or not [24–26]. Regarding GLP-1-RA, a meta-analysis suggests that this group not only reduces stroke, 3-point MACE, and mortality, but also significantly improves heart failure end points [27]. No randomized controlled trial data on heart failure risk are available on metformin, but a meta-analysis of nine cohort studies suggests that metformin was associated with a reduced risk of mortality and a small reduction in hospitalization for any reason in patients with heart failure compared with controls [40]. Regarding sulfonylureas, on the other hand, there is empirical evidence that they may be associated with an increased risk of heart failure events [42]. Thiazolidinediones (glitazones e.g., pioglitazone) are also not recommended for patients with heart failure based on current studies [28]. The same applies to DPP-4 inhibitors (especially saxagliptin and alogliptin). In contrast, linagliptin or sitagliptin can be used to lower blood glucose when GLP-1 RA is not indicated (BMI <28) or not tolerated [18–21].
£ HFpEF=sustained ejection fraction (EF ≥50%);
HFmEF=mid-range ejection fraction (HF=41-49%);
HFrEF=reduced ejection fraction (EF ≤40%)
| Insulin Dependence in Decreased GFR In patients treated with insulin, worsening renal function reduces insulin requirements and increases the risk of hypoglycemia. Therefore, insulin regimens and preparations that have the lowest risk of hypoglycemia should be preferred in the presence of decreased GFR. The nonsteroidal mineralocorticoid receptor antagonist finerenone has been shown to reduce functional decline by 22% in individuals with type 2 diabetes with chronic kidney disease. It also reduced the combined cardiovascular endpoint by 14%. |
| according to [4, 29-31] |
Check indication for insulin treatment
About a quarter of all T2D patients require insulin treatment. If insulin deficiency is the predominant factor at the onset of type 2 diabetes, the order of medications must be reversed; insulin must be administered in the first treatment step. However, patients in whom a triple combination of SGLT-2-i, GLP-1 RA, and metformin is not sufficient to achieve the HbA1c target are also indicated for insulin treatment. In the presence of decreased GFR, insulin regimens and preparations that have the lowest risk of hypoglycemia are preferable. When an ultra-long-acting basal insulin is used, the rate of hypoglycemia can be significantly reduced [14].
Furthermore, the updated SGED treatment recommendations include separate sections on T2D and NAFLD/NASH, as well as on the differential diagnosis of different diabetes subtypes.
Literature:
- “Diabetes Mellitus,” Medix, Last Revised: 02/2021, www.medix.ch/wissen/guidelines/diabetes-mellitus,(last accessed 04/25/2023).
- Hörber S, Heni M, Peter A: Laboratory diagnostics in people with diabetes [Laboratory diagnostics of people with diabetes]. Diabetologist 2022; 18(1): 77-86.
- American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes – 2021. Diabetes Care 2021;44:S15-S33.
- Gastaldi G, et al: Recommendations of the Swiss Society of Endocrinology and Diabetology (SGED/SSED) for the treatment of type 2 diabetes mellitus. 2023, www.sgedssed.ch, (last accessed 04/24/2023).
- Husain M, et al: PIONEER 6 Investigators. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2019; 381(9): 841-851.
- Mann JF, et al: LEADER Steering Committee and Investigators. Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med 2017; 377(9): 839-848.
- Marso SP, et al: SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2016; 375(19): 1834-1844.
- Marso SP, et al: LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2016; 375(4): 311-322.
- Neal B, et al: CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med 2017; 377(7): 644-657.
- Cannon CP, et al: VERTIS CV Investigators . Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N Engl J Med 2020; 383(15): 1425-1435.
- Gerstein HC, et al: REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394(10193): 121-130.
- Gerstein HC, et al: AMPLITUDE-O Trial Investigators . Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. N Engl J Med 2021 ;385(10): 896-907.
- Zinman B, et al: EMPA-REG OUTCOME Investigators . Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373(22): 2117-2128.
- Jensen MH, et al: Risk of Major Adverse Cardiovascular Events, Severe Hypoglycemia, and All-Cause Mortality for Widely Used Antihyperglycemic Dual and Triple Therapies for Type 2 Diabetes Management: A Cohort Study of All Danish Users. Diabetes Care. 2020;43(6): 1209-1218.
- Dave CV, et al: Risk of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Addition of SGLT2 Inhibitors Versus Sulfonylureas to Baseline GLP-1RA Therapy. Circulation 2021; 143(8): 770-779.
- Davies MJ, et al: Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022; 45(11): 2753-2786.
- Heerspink HJ, Langkilde AM, Wheeler DC: Dapagliflozin in Patients with Chronic Kidney Disease. Reply [Reply]. N Engl J Med 2021; 384(4): 389-390.
- Green JB, et al: TECOS Study Group . Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 373(3): 232-242.
- Rosenstock J, et al: CAROLINA Investigators. Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes: The CAROLINA Randomized Clinical Trial. JAMA 2019; 322(12): 1155-1166.
- Rosenstock J, et al: CARMELINA Investigators . Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019; 321(1): 69-79.
- Scirica BM, et al: SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369(14): 1317-1326.
- Boonman-de Winter LJ, et al: High prevalence of previously unknown heart failure and left ventricular dysfunction in patients with type 2 diabetes. Diabetologia 2012; 55(8): 2154-2162.
- Pop-Busui R, et al: Heart Failure: An Underappreciated Complication of Diabetes. A Consensus Report of the American Diabetes Association. Diabetes Care 2022; 45(7): 1670-1690.
- McMurray JJ, et al: DAPA-HF Trial Committees and Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019; 381(21): 1995-2008.
- Solomon SD, et al: DELIVER Trial Committees and Investigators . Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med 2022; 387(12): 1089-1098.
- Anker SD, et al: EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med 2021; 385(16): 1451-1461.
- Sattar N, et al: Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021; 9(10): 653-662.
- Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356(24): 2457-2471.
- Gæde P, et al: Years of life gained by multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: 21 years follow-up on the Steno-2 randomised trial. Diabetologia 2016; 59(11): 2298-2307.
HAUSARZT PRAXIS 2023; 18(5): 28-32