What should be considered in patients with chronic inflammatory skin diseases on immunomodulatory or immunosuppressive therapy? The Professional Association of German Dermatologists (BVDD) has published practical recommendations on this subject.
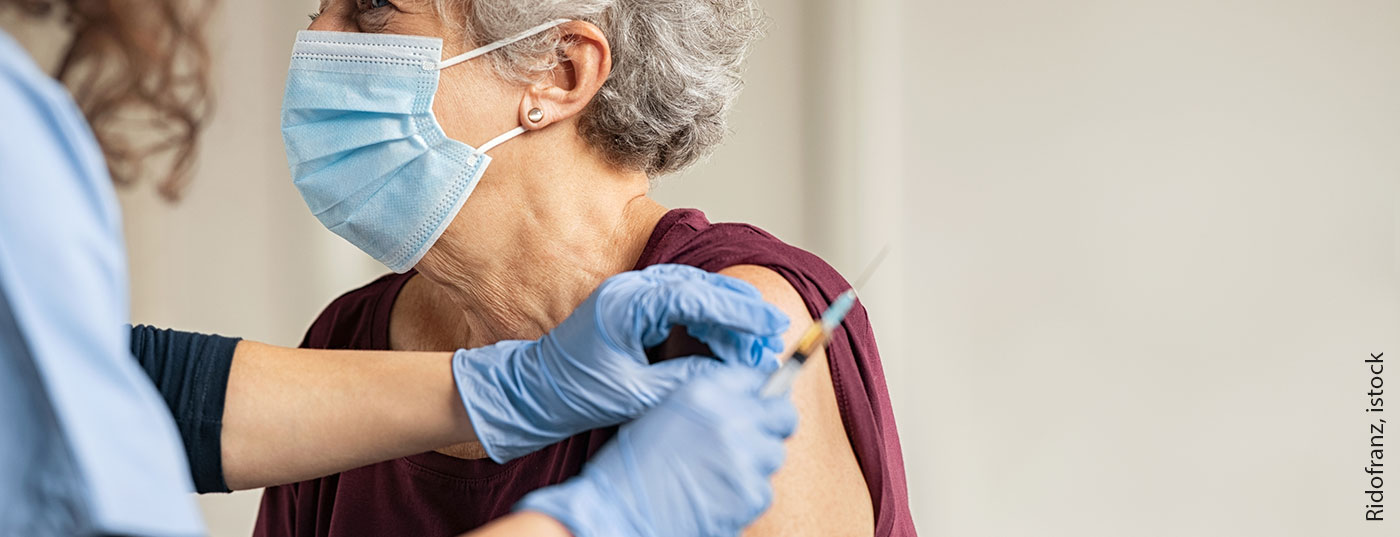
(red) The statement by Ralph von Kiedrowski, MD, BVDD board member, is intended to provide guidance regarding vaccination against SARS-CoV-2 in patients with chronic inflammatory skin diseases such as atopic dermatitis or psoriasis under systemic therapy. The following is an excerpt from the expert assessment published in this year’s January issue of the specialist journal Der Deutsche Dermatologe :
Within the framework of extensive registration studies of the (as of information Feb. 16, 2021) approved vaccine candidates from Biontech/Pfizer (Comirnaty, BNT162b2), Moderna (mRNA-1273), and AstraZeneca (AZD1222), a large number of individuals have already been vaccinated, but data on safety and efficacy in individuals with chronic inflammatory skin diseases or patients on immunomodulatory/immunosuppressive therapy are not yet available.
The known scientific findings apply: Dead vaccines are unrestrictedly suitable and applicable for patients with chronic inflammatory dermatoses and under immunomodulatory/immunosuppressive therapy. Classical dead vaccines are vaccines based on adjuvanted proteins. Vaccines based on non-replicable vectors (Astra-Zeneca) as well as vaccines based on mRNA technology (Biontech/Pfizer, Moderna) are also considered dead vaccines and therefore should not pose a risk to the patient population in dermatology practices.
An mRNA vaccine does not constitute a live vaccine. It is also not a “gene therapy”. An mRNA does not affect or change human DNA, but only provides a kind of building instruction for virus components (surface proteins, e.g. spike protein). The vaccinated person can thus temporarily produce virus protein components himself against which his own immune system can then form protective antibodies. Thus, like other inactivated vaccines, an mRNA vaccine is not a contraindication for patients on immunomodulatory/immunosuppressive therapy.
A vector vaccine, in which simian adenoviruses that are nonpathogenic to humans and incapable of replication express the spike protein on the surface, making it accessible to the immune system, also does not constitute a live vaccine. Theoretically, however, with the vector vaccine, immune reactions (as a side effect) against the carrier virus can occur with the second dose or the vaccine response can be diminished. This potential immunogenicity represents the most substantial difference from the mRNA variants.
|
Practical examples For methotrexate (MTX), Dr. von Kiedrowski recommends that the first corona shot be given two weeks after the last MTX application and the second three weeks later, and then continue MTX therapy after two (or four) more weeks. MTX is thus exposed seven to nine times. Biologics patients with a four-week application interval may receive their first corona vaccination two weeks after the last biologic, followed three weeks later by the second, and another two to four weeks later by continuation of therapy. The interruption of therapy due to vaccination is ultimately three to five weeks. For biologics such as ustekinumab, risankizumab, or tildrakizumab with a twelve-week interval, corona vaccination can be started six weeks after the last therapy injection, and the second vaccination would be given another three weeks later. This does not change the injection interval of the biologic (twelve weeks) (two-week safety interval) or the administration is postponed by one week (four-week interval). In view of the sometimes long-lasting efficacy of the various substances, a worsening of the underlying disease is rarely to be expected, according to Dr. von Kiedrowski. |
As a matter of principle, immunosuppression should be minimized for adequate vaccine response. Immunosuppressive agents play a minor role in patients with chronic inflammatory skin diseases (oral glucocorticosteroids, conditional ciclosporin A, long-acting B-cell depleting agents). According to the current state of knowledge, immunomodulatory agents do not impair the vaccination response, so that at the present time it cannot be recommended to change an existing immunomodulatory/immunosuppressive therapy because of a – with regard to SARS-CoV-2 currently also not yet generally available – vaccination.
In general, it should be noted: Under ongoing therapy, the recommendation is to administer the vaccination in the middle of a treatment interval and to continue therapy after two weeks at the earliest, and preferably after four weeks (box). Specific recommendations for therapies that must be applied weekly or even (multiple) daily do not exist. “My personal recommendation here is, depending on the disease activity, to let the periods after vaccination also apply before vaccination, i.e. to pause taking the vaccine for two to four weeks before vaccination,” says Ralph von Kiedrowski, MD.
Source: von Kiedrowski R: Corona vaccines suitable in immunomodulatory therapy. BVDD recommendations for vaccination against SARS-CoV-2. The German Dermatologist, 1. January 2021, 69(1): 16-17.
DERMATOLOGIE PRAXIS 2021; 31(1): 4 (published 2/25/21, ahead of print).