The approval of Dupilumab as the first biologic for the treatment of atopic dermatitis represents a significant advance. As a look at ongoing clinical trials shows, research has more arrows in its quiver. Among the biologics, the study programs for the IL13 antibody tralokinumab are the most advanced. Among the JAK inhibitors, baricitinib is a promising drug candidate which has already received an indication extension for atopic dermatitis in the EU.
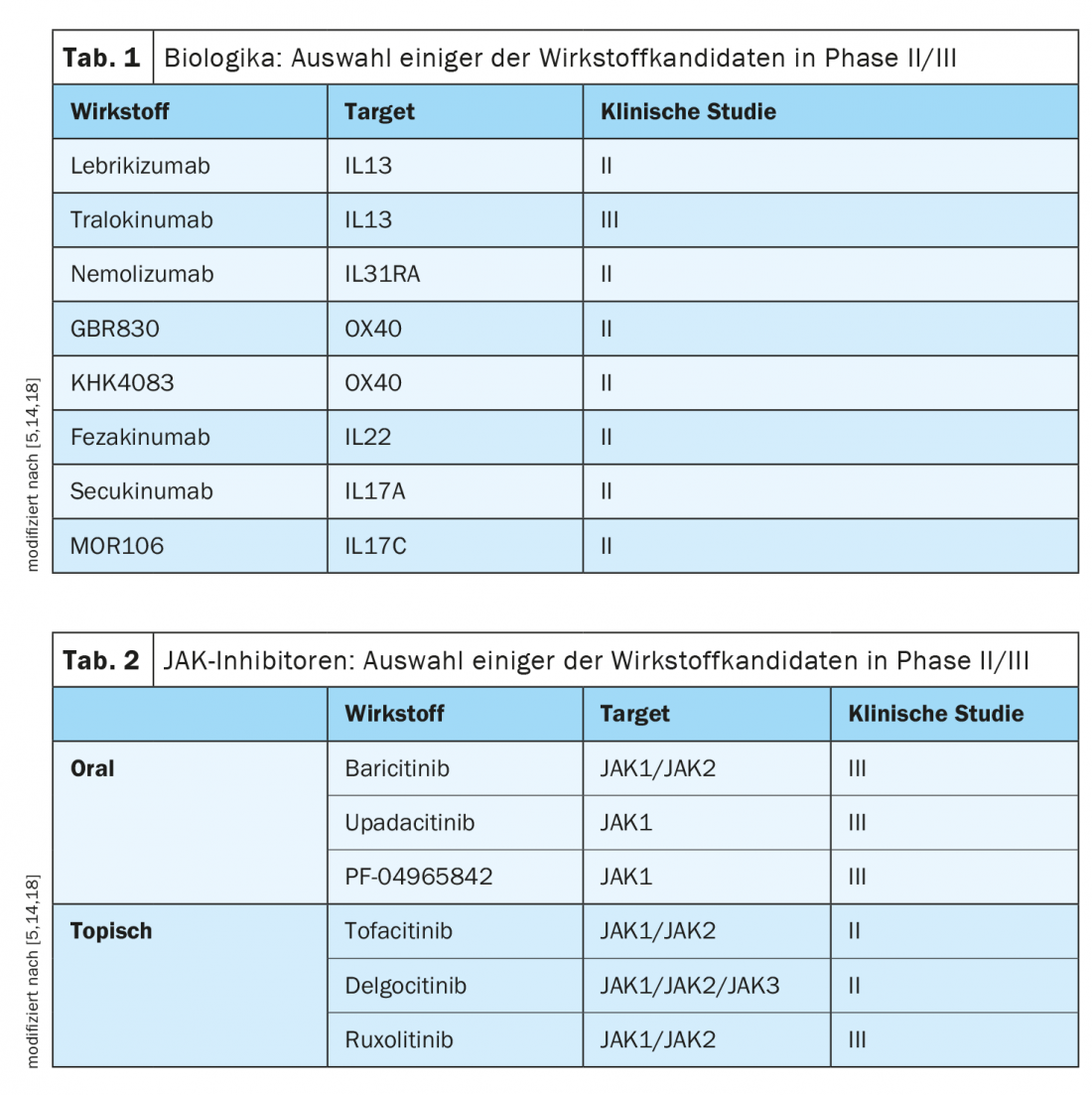
Further development of targeted therapeutic approaches is based in part on advances in understanding the complex and heterogeneous immunopathological basis of atopic dermatitis [1]. According to experts, the therapeutic landscape of this complex inflammatory disease will change rapidly in the coming years. There is a good chance that, in addition to dupilumab, other representatives of the biologics drug class will expand the treatment spectrum for moderate to severe forms of the disease in the not too distant future. (Tab.1). And in the field of JAK inhibitors, there are also several promising compounds being tested in clinical trials (Tab.2). In contrast to cortisone or ciclosporin, biologics and JAK inhibitors selectively affect individual activation cascades of the immune system. As a result, these modern therapeutic approaches not only have a high degree of efficacy, but also a favorable side-effect profile, which offers great advantages, particularly in view of the fact that atopic dermatitis is a chronic, relapsing clinical picture.
The biologic Dupilumab (Dupixent®), approved in Switzerland since 2019 for moderate to severe atopic dermatitis, has proven to be extremely effective and has an excellent benefit-risk profile. It is the first representative of the monoclonal antibodies for this indication. Dupilumab inhibits the signaling pathways of two key cytokines, IL4 and IL13, which are considered important drivers of the ongoing inflammatory process in atopic dermatitis [2,3]. That the results from large clinical trials such as SOLO1/2, CHRONOS or CAFE are also confirmed in practice is shown by data on the evaluation of this therapy option in the “real world” setting [2,4,5]. Patients benefit greatly from this treatment, which is also reflected in good adherence [5]. A look at ongoing clinical trials shows that, in addition to dupilumab, other targeted therapies could make a breakthrough in the next few years (tables 1 and 2).
In addition to tralokinumab, lebrikizumab also shows promising results
From studies of the immunopathological mechanisms in atopic dermatitis, it is clear that the cytokines IL4, IL13 as well as IL31 produced by Th2 cells have a central pathophysiological role [6]. Among the agents currently under clinical investigation, trials of the IL13 inhibitor tralokinumab are the most advanced [5]. In the phase III ECZTRA 1 and 2 studies, the tralokinumab condition (300 mg every 2 weeks) showed a significant improvement in IGA* compared to placebo (p<0.001) as early as 16 weeks [19]. This confirmed the good results of the phase IIb study, in which response rates of up to 70% for EASI50 and up to 40% for EASI75 were achieved in patients with moderate to severe atopic dermatitis [7].
* IGA = Investigators’ Global Assessment
|
Atopic dermatitis can lead to high levels of suffering The fact that atopic dermatitis is often associated with a considerable impairment of quality of life is shown by various empirical studies. In a population-based study of adults, more than half of those affected reported that the skin condition was associated with limitations in their daily lives, and for more than one-third, its appearance led to avoidance of social contact [15]. Moreover, these negative consequences were found to be significantly more pronounced in patients with moderate to severe atopic dermatitis than in those with mild courses. According to current data from health services research, approximately one in ten atopic dermatitis patients is affected by moderate to severe symptoms [16,17]. |
Similarly good efficacy data are available for lebrikizumab. This is also an IL13 inhibitor. Unlike tralokinumab, lebrikizumab prevents the binding of IL13R-α1 and IL4R-α-heterodimer receptor signaling complex, but not the binding of IL13 to the “decoy receptor IL13R-α2”, which can take up excess IL13 [8]. The results of the ongoing Phase III studies are still pending. In the two randomized placebo-controlled phase II studies conducted to date, patients treated with lebrikizumab showed a rapid and dose-dependent reduction in pruritus as well as a significant improvement with respect to EASI 50/75 and BSA [9,10]. Patients were randomized to the treatment arms of 125 mg every four weeks, 250 mg every four weeks, or 250 mg every two weeks for 16 weeks [11].
An interesting drug candidate, which targets IL31, is nemolizumab. Both proof-of-concept testing and longer observations in Phase IIb demonstrated added value in the area of pruritus control [12]. Further studies will show what the effect potential is with regard to other parameters. Also under clinical investigation are representatives of anti-OX40, anti-IL22 and anti-IL17 antibodies [5].
Baricitinib soon to be approved in Switzerland?
Unlike antibody-based therapies, which usually target cytokines or cytokine receptors, small molecules interfere with intracellular signaling pathways. The largest group of these small-molecule compounds are the Janus kinase (JAK) inhibitors. JAKs are intracellular enzymes that mediate the signaling cascade from a cytokine receptor into the cell. To date, various JAK inhibitors with different binding capacities to individual subtypes of Janus kinases (JAK1-3) have been developed. Baricitinib, a compound directed against JAK1/2, is one of the promising candidates in the field of oral JAK inhibitors [5]. Evaluations of the first phase II clinical trials report efficacy in the range of 60-82% with respect to EASI50 rates depending on treatment periods and study protocols [13]. In the EU, an indication extension for baricitinib has already been granted for atopic dermatitis [20]. In Switzerland, however, this treatment option is currently only approved for the indication of active rheumatoid arthritis. Promising data are also available on the JAK1 inhibitor upadacitinib. Results from interim analyses of a phase IIb study show good efficacy and tolerability at a dose of 30 mg/d in adult patients with moderate to severe atopic dermatitis [10].
In summary, JAK inhibitors for the treatment of atopic dermatitis have been shown to be effective in studies to date, with their rapid onset of action being of particular interest [5]. With regard to the side effect profile, it has been shown that intensified monitoring of patients is necessary to control the risk of infection and possible blood count changes. JAK inhibitors may be beneficial in the future, especially for short-term interval therapies [5]. The side effect profile varies depending on the substance and dose and can be reduced by topical application; several studies are currently underway (e.g., on tofacitinib, delgocitinib, ruxolitinib). The small molecular size of JAK inhibitors allows penetration through the skin, which is a good prerequisite for topical application.
Source: EADV Annual Meeting 2020
Literature:
- Langley RGB: Emerging Biologics for Atopic Dermatitis in Adults and Children, Professor Richard GB Langley MD, Halifax (Canada), EADV (Virtual) 10/31/2020.
- Simpson EL, et al: Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016; 375: 2335-2348.
- Swiss Drug Compendium, www.compendium.ch (last accessed Feb. 17, 2021).
- Thaci: Efficacy and safety of dupilumab monotherapy in adults with moderate-to-severe atopic dermatitis: a pooled analysis of two phase 3 randomized trials (LIBERTY AD SOLO 1 and LIBERTY AD SOLO 2). J Dermatol Sci 2019 266-275.
- Worm M, et al: Modern therapy of atopic dermatitis: biologics and small molecule drugs. JDDG 2020; 18: 10: 1085-1093.
- Bieber T, et al: Novel therapies based on the pathophysiology of atopic dermatitis. JDDG 2019 17; 11: 1150-1163.
- Wollenberg A, et al: Treatment of atopic dermatitis with tralokinumab, an anti-IL 13mAb. J Allergy Clin Immunol 2019; 143(1): 135-141.
- Maul JT: What’s new 2019/2020: inflammatory dermatoses. Julia-Tatjana Maul, MD, Zurich Dermatology Continuing Education Days (ZDFT), May 14-15, 2020.
- Simpson EL, Flohr C, Eichenfield LF, et al: Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol 2018; 78(5): 863-871.e811
- Guttman-Yassky E, et al: Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA Dermatol 2020: 156(4): 411-420.
- Loh TY, et al: Therapeutic Potential of Lebrikizumab in the Treatment of Atopic Dermatitis. J Asthma Allergy 2020; 13: 109-114.
- Ruzicka T, et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med 2017; 376: 826-835.
- Fragoulis GE, McInnes IB, Siebert S: JAK inhibitors. New players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology 2019; 58: i43-i54.
- Clinicaltrial.gov, www.clinicaltrial.gov (last accessed Feb. 17, 2021).
- Silverberg JI, et al: Patient burden and quality of life in atopic dermatitis in US adults A population-based cross-sectional study. Annals of Allergy, Asthma and Immunology 2018, DOI: https://doi.org/10.1016/j.anai.2018.07.006
- Augustin, et al: Data analysis CVderm 2020. care expertise neurodermatitis in Germany, data on file.
- Kirsten N: Neurodermatitis therapy during Corona: current concepts on the test bench and what is important for you now. Patient event, Dr. Natalia Kirsten, Hautnetz Hamburg, 17.09.20
- Li R, Hadi S, Guttman-Yassky E: Current and emerging biologic and small molecule therapies for atopic dermatitis. Expert Opin Biol Ther 2019; 4: 367-380.
- Wollenberg A: Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2), https://doi.org/10.1111/bjd.19574
- Hüttemann D: JAK inhibitors: New mode of action approved for atopic dermatitis. Pharmaceutical Newspaper (Online), 03.11.2020
DERMATOLOGIE PRAXIS 2021; 31(1): 33-34 (published 2/22/21, ahead of print).











