Many lung cancers are still diagnosed at a late or advanced and thus symptomatic stage. Based on recent study data, various professional societies have made positive recommendations for lung screening, but several questions remain unanswered for its implementation. In newly diagnosed lung cancer, accurate, non-invasive, preoperative staging should be performed immediately to ensure correct TNM classification, particularly as this provides the framework for appropriate therapy selection or treatment. represents the further patient management. Knowledge of the advantages, disadvantages, and limitations of the various noninvasive imaging modalities plays a significant role in this regard.
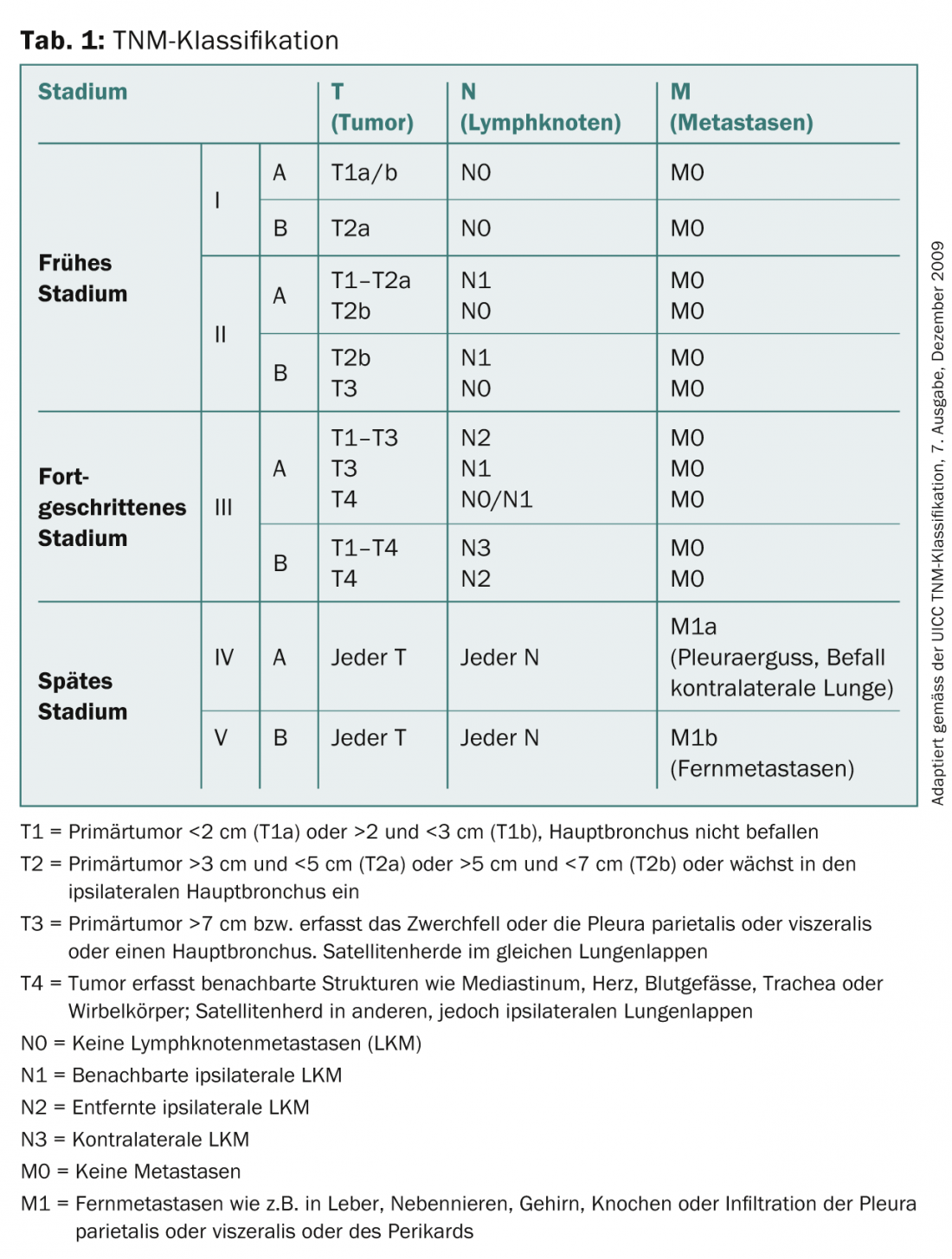
Lung cancer is the most common cause of cancer death in men and the second most common cause of cancer death in women in Switzerland. While new cases and mortality rates have been declining in men in recent years, they continue to increase in women. Despite significant advances in diagnostics and therapy, 5-year survival has stagnated, regardless of disease stage. It is approximately 14% in newly diagnosed lung cancer. When clinical symptoms occur, initial diagnosis is usually made by conventional radiography and computed tomography (CT) before a bioptic procedure for tissue typing and staging are followed. Imaging techniques such as CT, magnetic resonance imaging (MRI), integrated positron emission tomography/computed tomography (PET/CT), and bone scintigraphy are used to classify tumors according to the TNM classification (Table 1), on the basis of which the further therapeutic procedure is determined. This article evaluates the various radiologic diagnostic and staging tools along with their advantages, disadvantages, and limitations.
Since the publication of data from the National Lung Screening Trial [1] demonstrating that a targeted screening program using CT can reduce mortality, various professional societies have made positive recommendations for lung screening. However, various concerns and unresolved issues remain, e.g., regarding the handling and consequences of false positive findings, the recommendation and duration of control intervals, the risk of cumulative radiation exposure, the economic consequences for the health care system, and the regulation of financing [2].
Radiological diagnostics and staging
X-ray thorax: The X-ray of the thorax in two planes plays a minor role in the diagnosis due to its low sensitivity, since lung carcinoma cannot be excluded in the case of a negative finding. However, radiographs can be used to rule out or confirm other causes of thoracic discomfort. Often, imaging also serves as a baseline examination prior to surgery or therapy and is thus helpful in delineating treatment complications or non-tumor related disease during follow-up (Fig. 1).

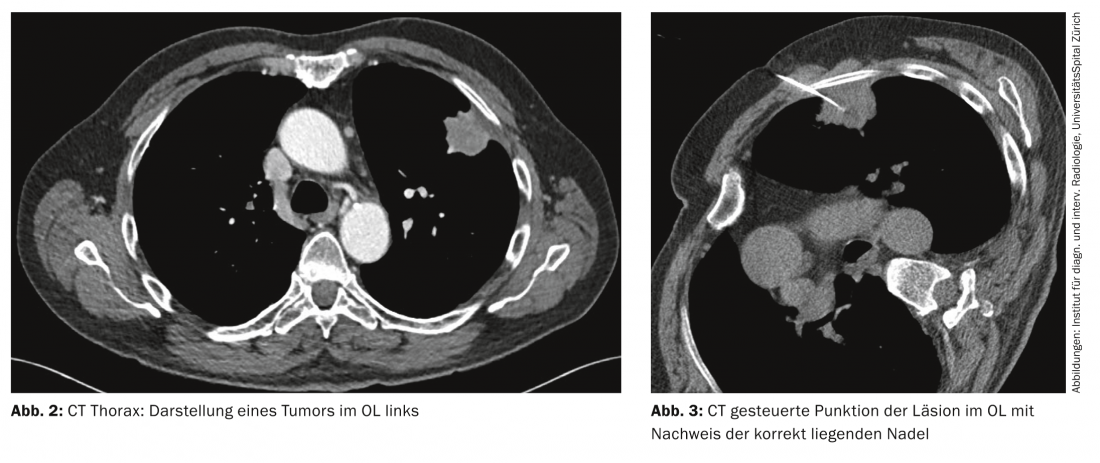
Computed tomography: Today, high-resolution CT of the thorax is the imaging modality of choice for the primary diagnosis of suspected lung carcinoma, especially due to its rapid availability (Fig. 2) .
Differentiation between a T1 and T2 tumor stage based mainly on tumor size is readily achieved by CT, but has little impact on further treatment strategy, whereas accuracy in differentiating between an operable T3 stage and a non-operable T4 stage is poor [3].
In nodal staging, CT has limited diagnostic accuracy with a sensitivity of 51% and a specificity of 86% [4], especially since it is based only on morphologic criteria such as shape and size, with a lymph node with a short-axis diameter >1 cm considered metastatic. Thus, the purely morphologic criteria such as size and shape can be applied to lymph node metastases, although they are ultimately insufficient for accurate diagnosis of mediastinal lymph node metastases and call for additional, more sensitive imaging techniques such as PET/CT.
In cases of suspected lung carcinoma, additional computed tomographic examination of the upper abdomen is recommended as part of primary imaging using contrast-enhanced CT of the thorax to evaluate for adrenal or liver metastases [4].
CT-guided biopsy is used to obtain tumor material for histological and immunohistochemical workup. It is particularly suitable for more peripherally located tumors (Fig. 3).
Magnetic resonance imaging: MRI can be used in conjunction with CT to assess the local extent of the tumor. be used for tumor infiltration of the mediastinum, thoracic wall, diaphragm, neuroforamina, and spinal canal, as well as the brachial plexus in Pancoast tumors. The technique of MR diffusion also allows further characterization of lung carcinoma and differentiation between central tumors and poststenotic lung atelectasis or consolidation.
In nodal staging of the mediastinum and pulmonary hili, MRI plays a secondary role and is mainly consulted as problem-solving imaging in unclear cases [5].
However, MRI is an effective imaging modality in the workup of brain or liver metastases [6]. MRI of the brain is recommended in patients with neurologic symptoms or, according to the NCCN guidelines [7], already in patients with a T2 tumor (stage Ib) who are scheduled for curative therapy, but should be performed safely from stage IIIA.
Integrated positron emission tomography/computed tomography: PET/CT with the tracer 18-fluoro-deoxy-glucose (FDG) provides important additional information in the preoperative staging of lung cancer due to its metabolic-functional and anatomical information. The use of PET/CT serves both to confirm initial computed tomographic staging and to detect computed tomographic false-negative thoracic lymph node metastases or occult thoracoabdominal distant metastases (Fig. 4).
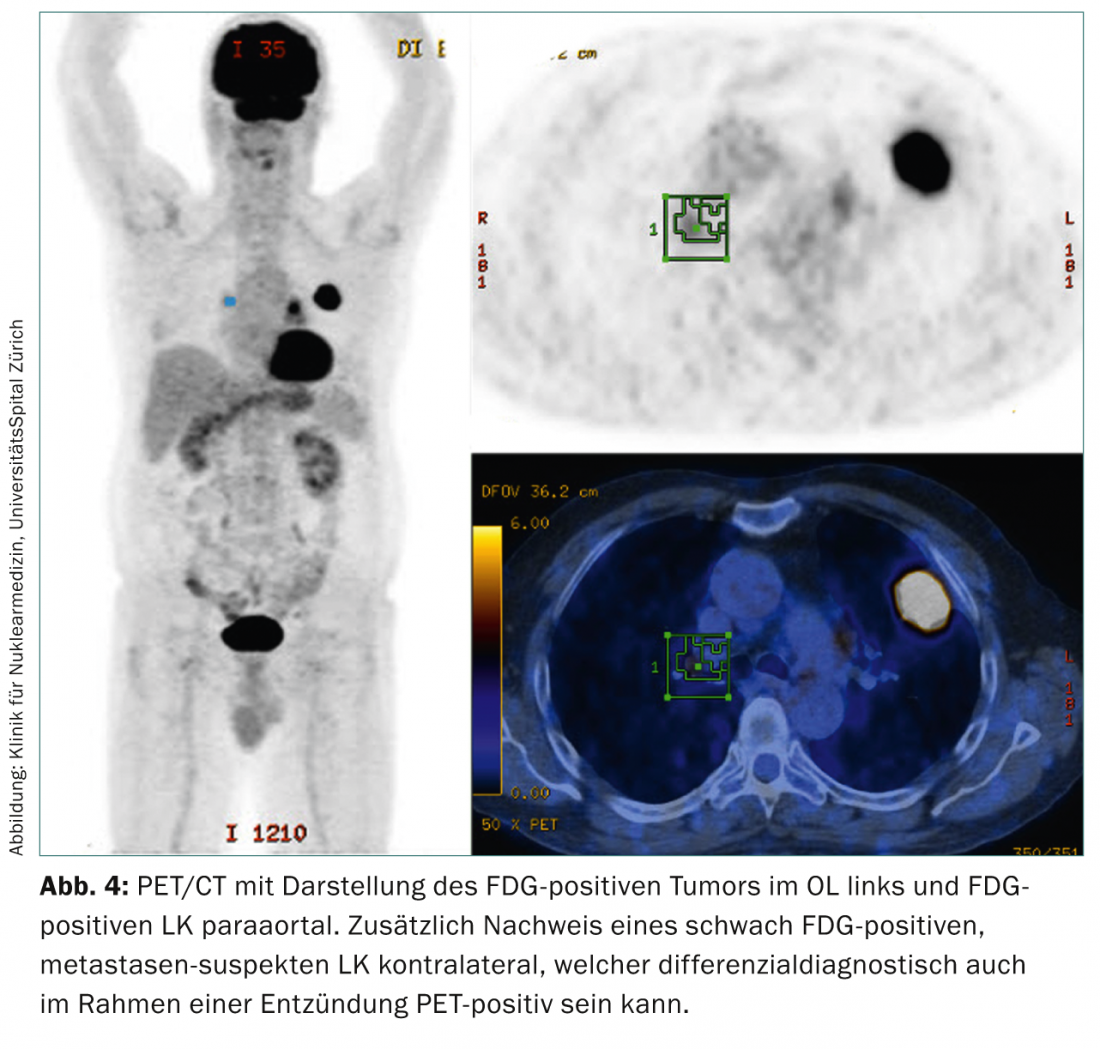
With its high negative predictive value of >90% [8], it leads to more accurate nodal staging than CT or PET alone. However, false positive findings can be caused by inflammation, infection, or infarction. False-negative findings may also occur, which must then be further clarified by invasive diagnostics to confirm computed tomographic staging of enlarged thoracic lymph nodes.
In the context of metastasis search, PET/CT allows detection of unexpected thoracoabdominal distant metastases and can thus reduce the rate of unnecessary resp. therapeutically not indicated thoracotomies by up to 20% [9]. In the clarification of possible brain metastases, FDG-PET/CT is not suitable due to the physiologically high glucose metabolism of the brain and is clearly inferior to MRI here.
Bone scintigraphy: Current studies show that FDG-PET/CT has comparable sensitivity and accuracy to bone scintigraphy, but with improved specificity due to the significantly lower rate of false positive findings, which is up to 40% for bone scintigraphy. Thus, bone scintigraphy plays a minor role in the preoperative staging of lung cancer.
Non-invasive staging
T stage: CT is the primary method used. It allows clear differentiation between T1 and T2 but has weaknesses in differentiating between T3 and T4. Here, MRI should be considered as an alternative method.
N stage: PET/CT is the method of choice. It has high sensitivity but cannot differentiate between tumor and inflammation/infection. CT has high specificity but low sensitivity.
M stage: At this stage, PET/CT is the method of choice. From tumor stage T3, MRI of the brain is recommended to exclude cerebral metastases. Bone scintigraphy plays a minor role in the detection of bone metastases.
Aftercare
Currently, there is still no clear procedure for the follow-up of curatively treated tumors. The ACCP (“American College of Chest Physician”) guidelines for non-small cell lung cancer require follow-up by chest CT every six months for the first two years and annual follow-up thereafter [10]. In patients in whom the tumor has not received primary curative treatment, recommendations are based on the type of therapies but usually provide for a three-month course.
PD Thomas Frauenfelder, MD
Stephan Baumüller, MD
Literature:
- Aberle DR, et al: National Lung Screening Trial Research T: Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011; 365(5): 395-409.
- Bach PB, et al: Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012; 307(22): 2418-2429.
- Quint LE, et al: Preoperative staging of non-small-cell carcinoma of the lung: imaging methods. AJR Am J Roentgenol 1995; 164(6): 1349-1359.
- Ravenel JG, et al: ACR Appropriateness Criteria(R) noninvasive clinical staging of bronchogenic carcinoma. J Thorac Imaging 2010; 25(4): W107-111.
- Imai K, et al: Diagnostic imaging in the preoperative management of lung cancer. Surg Today 2013.
- Tsim S, et al: Staging of non-small cell lung cancer (NSCLC): a review. Respir Med 2010; 104(12): 1767-1774.
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Non-small Cell Lung Cancer, version 1.20142014. Available at: www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- Vansteenkiste J, et al: Positron-emission tomography in prognostic and therapeutic assessment of lung cancer: systematic review. Lancet Oncol 2004; 5(9): 531-540.
- van Tinteren H, et al: Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 2002; 359(9315): 1388-1393.
- Rubins J, et al: Follow-up and surveillance of the lung cancer patient following curative intent therapy: ACCP evidence-based clinical practice guideline (2nd edition). Chest 2007; 132(3): 355-367.
InFo ONCOLOGY HEMATOLOGY 2014; 2(4): 6-9.