The history of stents is marked by numerous successes, but also setbacks. In the meantime, many “teething troubles” have been overcome. A wide range of stents is available for different situations.
Coronary heart disease (CHD) is the sad winner worldwide when it comes to causes of death, according to PD Stefan Blöchlinger, MD, PhD, from the Department of Cardiology at Winterthur Cantonal Hospital. The first percutaneous transluminal coronary angioplasty, or PTCA or PCI, was performed in Zurich on September 16, 1977, in a 38-year-old patient with isolated proximal RIVA stenosis. Less than two years later, the experience with the new procedure was published in the New England Journal of Medicine [1]. However, balloon angioplasty has some inherent problems. So-called elastic recoil of the artery, restenosis and thrombus formation are possible. In order to reduce restenosis, prevent collapse of the vessel wall or To prevent dissection with thrombus formation as far as possible and thus keep the vessel open, coronary stents were finally developed a few years later. Via catheter, the “scaffolds” enter the occluded vessel, which should hold the deposits to the arterial wall, permanently dilate the artery, and improve blood flow [2]. However, with the so-called bare-metal stents (BMS), restenosis had by no means disappeared, which is related, among other things, to intimal hyperplasia, in which proliferation and migration of smooth muscle cells occur in the area of the stents. Thrombosis also remained a threat due to the thrombogenicity of the metal and damage to the endothelium.
So what happened next?
The so-called drug-eluting stents (DES) should provide a remedy. Via controlled local delivery of antiproliferative agents, these could actually improve the outcome of stent implantation and significantly reduce the reintervention rate. However, one problem remained or, more precisely, had even worsened with the first generations of drug-eluting stents: the occurrence of very late stent thrombosis, i.e., more than one year after implantation [3,4]. With an incidence of about 0.5% per year, the older models were even inferior to the BMS in this respect. It was not until the introduction of the next generation of DES that this problem was finally mastered and the incidence rate was reduced to 0.1% per year. Moreover, the newer DES generation is only coated with agents ending in -limus (everolimus, zotarolimus and sirolimus). A meta-analysis had shown: A coating with sirolimus is again clearly superior to that with paclitaxel with regard to restenosis rate [5].
Bioresorbable stents – the next step?
The most recent development is bioresorbable DES or “scaffolds” represent. The best known representative is called ABSORB. However, modern metal stents are so technically advanced that they are actually hard to beat in terms of effectiveness and safety. Their retention in the body does not presently pose a burning clinical problem. Bioresorbable stents were therefore developed not so much to overcome serious disadvantages of the existing newer generation stents, but rather from the idea that the native state of the vessel was superior or preferable to a lifelong “metal cage”. The ABSORB studies, however, were disappointing and even showed a worsening of safety: among other things, the (very late) risk of thrombosis was significantly increased compared to the non-absorbable DES [6], which was probably mainly due to scaffold discontinuity, followed by malapposition and neoatherosclerosis [7].
Therefore, bioresorbable stents should not be preferred to metal DES of the newer generation in clinical practice at present. Patients who have already received bioresorbable stents should continue dual antiplatelet therapy (DAPT) for the presumed duration of resorption if well tolerated (i.e., at least 36 months for ABSORB) or, if previously stopped, restart it depending on the individual case and thrombosis-bleeding risk [8].
Guidelines
What do the guidelines say about the different types of stents? With regard to BMS, the current European guidelines on myocardial revascularization of 2014 are relatively clear: BMS would actually no longer have a place in this field, i.e., there would be no indication for it, regardless of lesion type or patient – this mainly due to their “Achilles heel”, restenoses. Clear evidence on differences between BMS and DES with regard to the risk of stent thrombosis in unplanned DAPT interruption was not available [9]. Further specific recommendations are shown in Table 1. In general, DES of the newer generation are recommended.
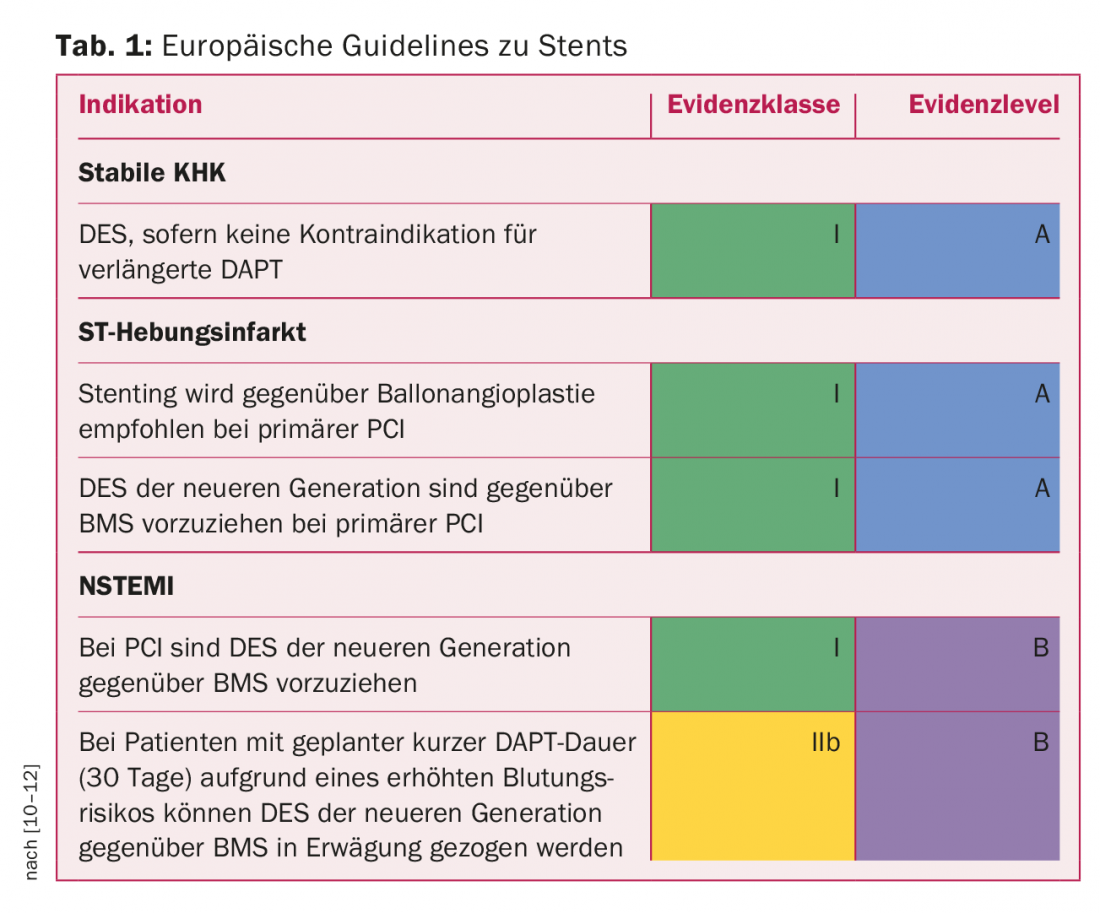
Dr. Blöchlinger also sees only a few special cases where BMS can still be an option (e.g. very large vessel diameters, as DES of this size are currently not available). So-called covered stents also belong to the BMS. These stents are lined with graft material, they are mainly used for problems such as vascular rupture during coronary interventions. At the moment, there are five different such stents in Europe.
Source: 16th Zurich Review Course in Clinical Cardiology, April 12-14, 2018, Zurich Oerlikon.
Literature:
- Grüntzig AR, Senning A, Siegenthaler WE: Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med 1979 Jul 12; 301(2): 61-68.
- Carrié D, et al: Ten-year clinical and angiographic follow-up of coronary wallstent. Am J Cardiol 2000 Jan 1; 85(1): 95-98, A8.
- Stefanini GG, et al: Long-term clinical outcomes of biodegradable polymer biolimus-eluting stents versus durable polymer sirolimus-eluting stents in patients with coronary artery disease (LEADERS): 4 year follow-up of a randomised non-inferiority trial. Lancet 2011 Dec 3; 378(9807): 1940-1948.
- Wenaweser P, et al: Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice. 4-year results from a large 2-institutional cohort study. J Am Coll Cardiol 2008 Sep 30; 52(14): 1134-1140.
- Stettler C, et al: Outcomes associated with drug-eluting and bare-metal stents: a collaborative network meta-analysis. Lancet 2007 Sep 15; 370(9591): 937-948.
- Ali ZA, et al: Three-Year Outcomes With the Absorb Bioresorbable Scaffold: Individual-Patient Data Meta-Analysis From the ABSORB Randomized Trials. Circulation 2018 Jan 30; 137(5): 464-479.
- Yamaji K, et al: Mechanisms of Very Late Bioresorbable Scaffold Thrombosis: The INVEST Registry. J Am Coll Cardiol 2017 Nov 7; 70(19): 2330-2344.
- Byrne RA, et al: Report of an ESC-EAPCI Task Force on the evaluation and use of bioresorbable scaffolds for percutaneous coronary intervention: executive summary. Eur Heart J 2017 Aug 28. DOI: 10.1093/eurheartj/ehx488. [Epub ahead of print].
- Windecker S, et al: 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014 Oct 1; 35(37): 2541-2619.
- Montalescot G, et al: 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013 Oct; 34(38): 2949-3003.
- Ibanez B, et al: 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018 Jan 7; 39(2): 119-177.
- Roffi M, et al: 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016 Jan 14; 37(3): 267-315.
CARDIOVASC 2018; 17(3): 33-34











