Pulmonary hypertension in left heart disease is most commonly caused by passive pressure elevation due to increased left-sided filling pressures. While isolated postcapillary pulmonary hypertension is common, only a small group of patients is affected by severe pulmonary vascular disease.
While idiopathic pulmonary arterial hypertension remains a zebra in the broad field of pulmonary hypertension (PH), in 65-80% of patients with PH, left heart disease is present as the cause of the increase in small circuit pressure (hereafter abbreviated as PH-LHD for Left Heart Disease) [20]. Leading clinicians and researchers in the field of PH differentiate between PH-LHD with a left ventricular phenotype and PH-LHD with a right ventricular phenotype, with left ventricular type referring to the presence of isolated postcapillary PH (IpcPH) and right ventricular type referring to the presence of combined postcapillary and precapillary PH (CpcPH). While IpcPH is common, only a small group of patients (<10%) are affected by severe pulmonary vascular disease (CpcPH). The transition from left to right ventricular phenotypes is thought to be smooth and caused by so-called remodeling of the small pulmonary arteries of the pulmonary vascular bed. This review will take a closer look at the diagnostic criteria, their limitations and inaccuracies, and possible therapeutic options for PH-LHD.
Definition, pathophysiology and diagnostics
PH is a common, prognostically relevant problem in patients with left heart disease and is associated with reduced exercise capacity, and increased morbidity and mortality [15]. According to current guidelines, PH-LHD is assigned to WHO group 2 of a total of 5 PH groups (group 1: pulmonary arterial hypertension (PAH), 2: PH in left heart disease, 3: PH in pulmonary disease or hypoxia, 4: chronic thromboembolic PH, 5: PH with unclear attribution or multifactorial mechanisms) [7]. Initially, PH-LHD results from passive, so-called postcapillary, backpressure of the left-sided – especially the left atrial – filling pressures. This backwater is associated with increased permeability of the alveolocapillary membrane and can cause interstitial and alveolar pulmonary edema. PH-LHD may occur in patients with left heart failure with reduced (HFrEF), as well as with preserved pump function (HFpEF) or valvular defects. The prevalence of PH in HFrEF is reported between 40-75% [8,17] and in HFpEF between 36-83% [13,22].
It is suggested that in some patients – in terms of a temporal pathophysiological sequence – the permanent, passive increase in pressure in the pulmonary circulation may lead to (irreversible) remodeling of the pulmonary arterial vessels and thus to pulmonary vascular disease and ultimately right heart failure. However, it is unclear in which patients this occurs and in which it does not. As with many diseases, a genetic predisposition is suspected in addition to environmental influences, but has not yet been proven.
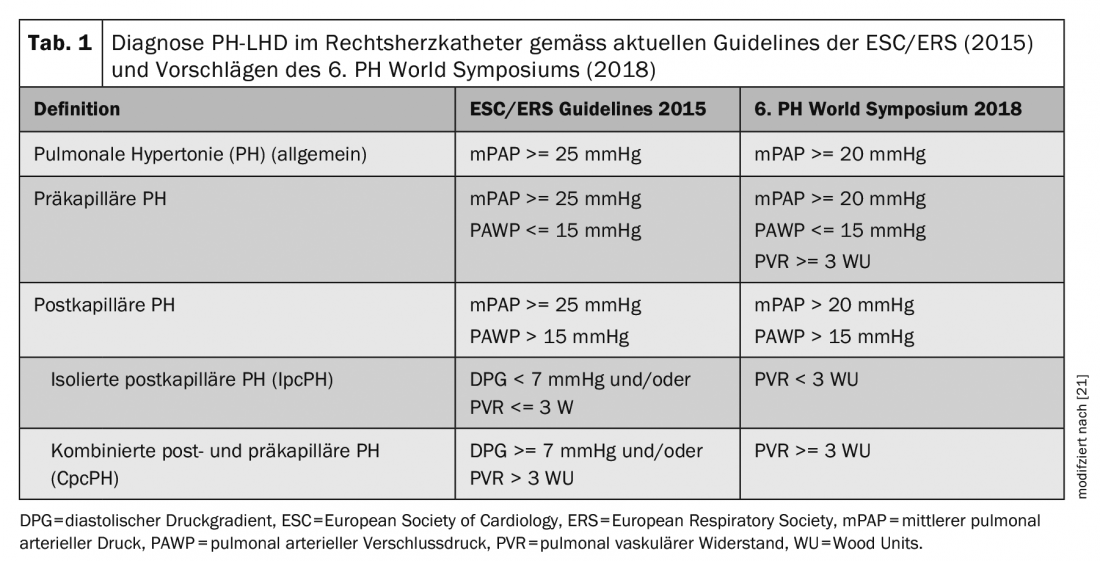
According to current guidelines [7], postcapillary PH is diagnosed when a mean pulmonary arterial pressure (mPAP) >= 25 mmHg and pulmonary arterial occlusion pressure (PAWP) >15 mmHg are measured during right heart catheterization. To further differentiate between IpcPH and CpcPH, diastolic pressure gradient (diastolic PAP – PAWP) and pulmonary vascular resistance (PVR = (mPAP-PAWP)/Cardiac Output) are also determined. See Table 1, where the new diagnostic criteria as proposed by the 6th PH World Symposium [25] are also listed. Studies of more than 20 000 subjects have shown that an mPAP between 21-24 mmHg is associated with increased mortality regardless of PH group-even in patients with left heart failure [3,5,16]. The normal value of mPAP is (mean +/- standard deviation) 14 +/- 3 mmHg. Overall, then, these studies showed that above the threshold of mean +2 standard deviations, there is disease value, which is why the current recommendations for diagnosing PH were adjusted during the PH World Symposium from mPAP >= 25 mmHg to >20 mmHg.
Not every patient needs a right heart catheter
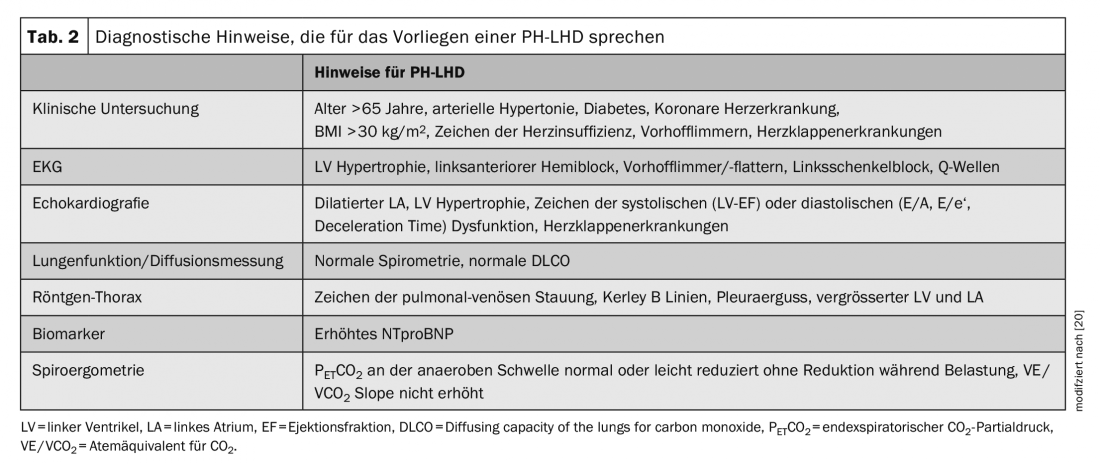
If a patient has a history of PH, noninvasive testing is recommended first to more accurately narrow down the cause and establish a working hypothesis. Due to the complexity of the subject, only the differentiation between a PAH and PH-LHD will be discussed here and the other groups of PH (groups 2, 4, and 5) will be neglected, although these important differential diagnoses should not be forgotten in the workup of a PH. The recommended tests and results indicative of PH-LHD are listed in Table 2.
If there is echocardiographic evidence of pulmonary vascular disease, namely an elevated RV/LV ratio >= 1.0, LV centricity index >1.2, apex formation through the right ventricle, vena cava diameter > 2 cm without collapse and an estimated systolic PAP >50 mmHg, right heart catheterization should be performed for further differentiation, even if the signs of PH-LHD listed in Table 2 are present. If only mild PH or none of the echocardiographic signs of pulmonary vascular disease are present, it is most likely postcapillary PH and further invasive evaluation is not indicated.
Limitations and difficulties of right heart catheterization.
Both for the diagnosis of PH-LHD per se and the differentiation of a possible precapillary component, the correct measurement of PAWP is essential. There are limitations and inaccuracies here that the investigator should be aware of and take into account when interpreting the results.
PAWP measurement is not feasible in every patient (the “wedge position” cannot be achieved if dilated pulmonary arteries are present, for example). In these cases, especially when PH-LHD is highly suspected, direct measurement of LVEDP (left ventricular end-diastolic pressure) in the left heart catheter is recommended.
Incorrect determination of the zero point can also lead to measurement errors. The zero point should be mid-thoracic at the level of the left atrium [11]. In the presence of a thoracic deformity, determination of the zero point can be extremely difficult.
There is disagreement among experts regarding the measurement of PAWP. While many cardiologists prefer the time point at the end of normal expiration for resting measurements, PH experts tend to believe that the curves of PAWP should also be averaged over several respiratory cycles, especially also to be able to compare resting values with values under stress [12]. Breath holding or forced breathing leads to falsification of the values (intrathoracic pressure, Valsalva etc). In patients with lung diseases, e.g. COPD, the pressure curves show prominent respiratory fluctuations. In these cases, averaging the pressure curves over several breathing cycles is recommended.
There is also a risk of erroneous PAWP measurement in the presence of atrial fibrillation or a high v wave as an indication of mitral regurgitation. Abdominal obesity can also confound readings by increasing intra-abdominal and intrathoracic pressures.
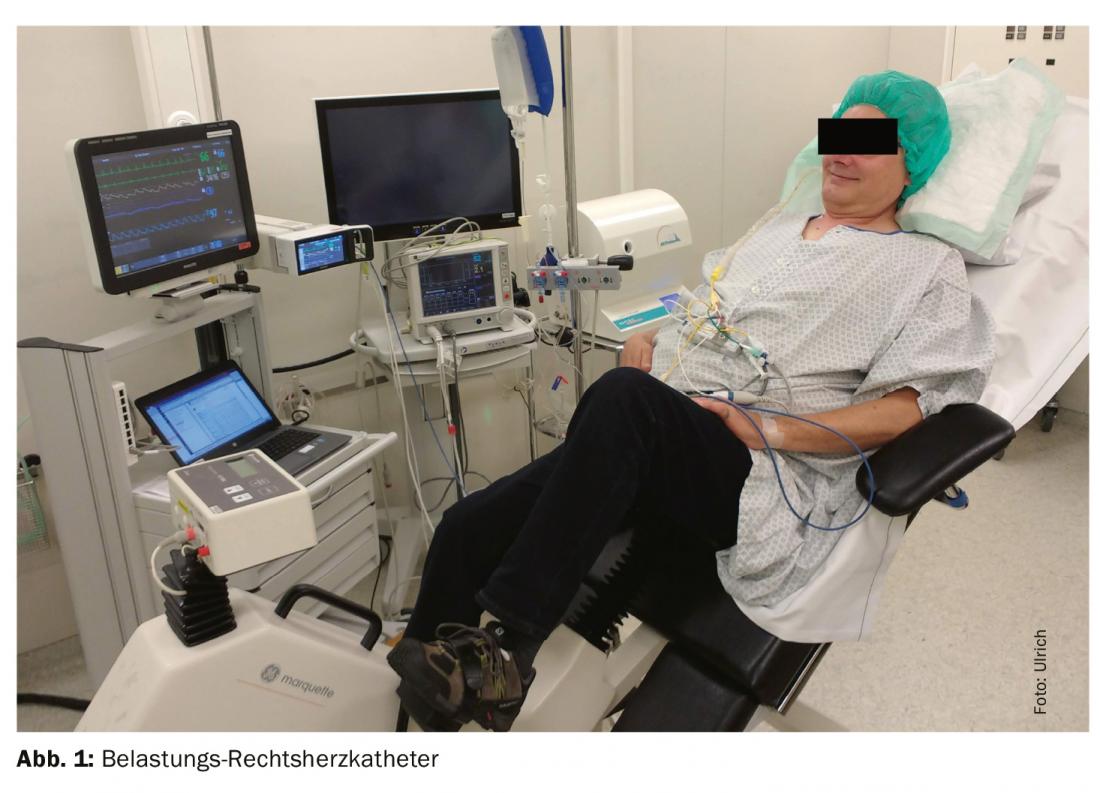
Finally, right heart catheterization should be performed only in compensated disease state, i.e., in the absence of cardiac water retention. In the well-controlled patient in normovolemia, hydrostatic pressure in the small circulation should be normal and PH at rest should not occur. Nevertheless, a patient who is too “dry” may also have false-deep PAWP pressures, and thus postcapillary PH may be misclassified as precapillary. To unmask occult PH-LHD, supplemental stress testing by volume administration or exercise testing (bicycle ergometry, Fig. 1) is recommended, especially for borderline PAWP pressures (13-15 mmHg). However, standardized and validated measurement methods are not yet available [25]. Because these examinations and interpretation of the result can be complex, referral to a tertiary center with experience in PH is recommended.
Therapy
Because most cases of PH-LHD are passive, postcapillary PH, the primary therapeutic goal of all PH-LHD is optimal heart failure therapy and normalization of filling pressures. These include intensive diuretic therapy, drug therapy for heart failure, and interventional procedures (CRT, ICD, LVAD, MitraClip, and others).
HFrEF: According to current guidelines [19], drug therapy with ACE inhibitors/angiotensin II receptor antagonists, neprilysin inhibitors, beta-blockers, and mineral coroid receptor antagonists is recommended. Above all, it is crucial to achieve the intended target dosages and good therapy adherence. Initial study results with the SGLT2 inhibitor dapagilfozin show promise [27]. In addition, emphasis is placed on adequate heart rate control, which can be supplemented with ivabradine if beta-blockers are not effective. If drug therapy is not sufficient, cardiac resynchronization therapy may be indicated.
HFpEF: Therapy of HFpEF remains a challenge. To date, there are no good, evidence-based therapies for which therapeutic success has been demonstrated. The recent PARAGON-HF trial (valsartan vs Entresto) also failed to achieve a significant primary endpoint [23]. Basically, with the current state of knowledge for the therapy of left ventricular stiffness and diastolic dysfunction, optimization of blood pressure control and intensive diuretic therapy to control volume balance are recommended. The exclusion of cardiac amyloidosis should also be considered in the presence of HFpEF.
Functional mitral regurgitation: If functional mitral regurgitation due to dilated left-sided heart cavities is present, it is not only associated with increased mortality [4], but should also be considered as a possible causative factor of PH. Significant improvement in hemodynamics in patients with HFrEF has been demonstrated after mitral valve repair [6]. Nevertheless, it is important to note that patients with PH have increased mortality rates with mitral clip interventions [24].
Left ventricular assist device (LVAD): The LVAD should also be briefly mentioned here, as it is a therapeutic option in advanced HFrEF. By increasing left ventricular ejection, the LV is unloaded, thereby aiming for normalization of hemodynamics. Normalization of pulmonary hemodynamics was achieved in 30% of patients with fixed PH (i.e., pulmonary vascular component) with the use of LVAD and inotropics before cardiac transplantation [2].
Implantable pressure sensor – CardioMEMS: The use of implantable pressure sensors has demonstrated that “hemodynamic” decompensation occurs approximately 2-3 weeks before clinical decompensation. Targeted monitoring of pulmonary pressure using CardioMEMS significantly reduced the rate of hospitalizations in patients with HFpEF and HFrEF with early drug interventions (diuretics) [1].
Targeted PH therapy: existing therapies for PH (endothelin receptor antagonists (ERA), prostanoids, phosphodiesterase-5 inhibitors (PDE-5 inhibitors), soluble guanylate cyclase stimulators (sGC)) are not approved for PH-LHD. Current guidelines also generally do not recommend targeted PH therapy in patients with PH-LHD (III-C) [7].
Nevertheless, in selected cases, when a significant pulmonary vascular component with markedly elevated PVR is present, a therapeutic trial with targeted PH medication may be attempted (IIa-C recommendation). These considerations are mainly based on small case series and registry data on the PDE-5 inhibitor sildenafil, which has been shown to reduce PVR and improve right ventricular function in patients with CpcPH, among other outcomes [9,10,14,26], and results from the European Compera Registry [18]. After all, the rationale behind this is that concomitant rare IPAH (zebra) may be present along with common heart failure (horse).
In contrast, several large randomized trials have demonstrated neutral results or even worsening outcomes with targeted PAH therapies in unselected patients with left ventricular failure without a major PH component. A summary of recently completed and ongoing studies can be found in the recent PH Worldsymposium comments [25]. The biggest criticism of the studies completed to date is a lack of clear hemodynamic differentiation, largely including patients with IpcPH and partially including patients with HFpEF without PH. A deleterious effect of PH medication in patients with isolated postcapillary PH is thought to be due to pulmonary vasodilation attempted by the drug effect with a consecutive increase in pulmonary blood flow, which may lead to pulmonary edema and cardiac decompensation in patients with preexisting left-sided elevated filling pressures.
In summary, in patients with isolated postcapillary PH (IpcPH), targeted PH therapy should not be used and may even be harmful. Patients with CpcPH who might benefit from therapy should be referred to a PH center where they can be evaluated by experts and treated, ideally in the context of trials.
Take-Home Messages
- Pulmonary hypertension in left heart disease (PH-LHD) is common and in most cases is due to passive pressure elevation from increased left-sided filling pressures.
- If a precapillary component/pulmonary vascular disease is suspected, further evaluation by right heart catheterization should be performed.
- Specific PH therapy may be considered by the expert at the PH center in patients with significant precapillary components, as the distinction from rare idiopathic pulmonary arterial hypertension may be difficult.
Literature:
- Abraham WT, Stevenson LW, Bourge RC, et al: Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet 387: 453-461, 2016.
- Al-Kindi SG, Farhoud M, Zacharias M, et al: Left Ventricular Assist Devices or Inotropes for Decreasing Pulmonary Vascular Resistance in Patients with Pulmonary Hypertension Listed for Heart Transplantation. Journal of cardiac failure 23: 209-215, 2017.
- Assad TR, Maron BA, Robbins IM, et al: Prognostic Effect and Longitudinal Hemodynamic Assessment of Borderline Pulmonary Hypertension. JAMA Cardiology 2: 1361-1368, 2017.
- Bursi F, Barbieri A, Grigioni F, et al: Prognostic implications of functional mitral regurgitation according to the severity of the underlying chronic heart failure: a long-term outcome study. 12: 382-388, 2010.
- Douschan P, Kovacs G, Avian A, et al: Mild Elevation of Pulmonary Arterial Pressure as a Predictor of Mortality. 197: 509-516, 2018.
- Gaemperli O, Moccetti M, Surder D, et al: Acute haemodynamic changes after percutaneous mitral valve repair: relation to mid-term outcomes. 98: 126-132, 2012.
- Galie N, Humbert M, Vachiery JL, et al: 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). European heart journal 37: 67-119, 2016.
- Ghio S, Gavazzi A, Campana C, et al: Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. Journal of the American College of Cardiology 37: 183-188, 2001.
- Guazzi M, Vicenzi M, Arena R, Guazzi MD: Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation 124: 164-174, 2011.
- Hoendermis ES, Liu LC, Hummel YM, et al: Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. European heart journal 36: 2565-2573, 2015.
- Kovacs G, Avian A, Pienn M, et al: Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. American journal of respiratory and critical care medicine 190: 252-257, 2014.
- Kovacs G, Herve P, Barbera JA, et al: An official European Respiratory Society statement: pulmonary haemodynamics during exercise. The European respiratory journal 50: 2017.
- Lam CS, Roger VL, Rodeheffer RJ, et al: Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. Journal of the American College of Cardiology 53: 1119-1126, 2009.
- Lewis GD, Shah R, Shahzad K, et al: Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation 116: 1555-1562, 2007.
- Luscher TF: Pulmonary embolism and pulmonary hypertension: two issues often neglected in cardiology. European heart journal 36: 581-583, 2015.
- Maron BA, Hess E, Maddox TM, et al: Association of Borderline Pulmonary Hypertension With Mortality and Hospitalization in a Large Patient Cohort: Insights From the Veterans Affairs Clinical Assessment, Reporting, and Tracking Program. Circulation 133: 1240-1248, 2016.
- Miller WL, Grill DE, Borlaug BA: Clinical features, hemodynamics, and outcomes of pulmonary hypertension due to chronic heart failure with reduced ejection fraction: pulmonary hypertension and heart failure. JACC Heart failure 1: 290-299, 2013.
- Opitz CF, Hoeper MM, Gibbs JS, et al: Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. Journal of the American College of Cardiology 68: 368-378, 2016.
- Ponikowski P, Voors AA, Anker SD, et al: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 18: 891-975, 2016.
- Rosenkranz S, Gibbs JS, Wachter R, et al: Left ventricular heart failure and pulmonary hypertension. European heart journal 37: 942-954, 2016.
- Rosenkranz S, Kramer T, Gerhardt F, et al: Pulmonary hypertension in HFpEF and HFrEF: Pathophysiology, diagnosis, treatment approaches. Heart 2019.
- Shah AM, Shah SJ, Anand IS, et al: Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circulation Heart failure 7: 104-115, 2014.
- Solomon SD, McMurray JJV, Anand IS, et al: Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. The New England journal of medicine 2019.
- Tigges E, Blankenberg S, von Bardeleben RS, et al: Implication of pulmonary hypertension in patients undergoing MitraClip therapy: results from the German transcatheter mitral valve interventions (TRAMI) registry. 20: 585-594, 2018.
- Vachiery JL, Tedford RJ, Rosenkranz S, et al: Pulmonary hypertension due to left heart disease. The European respiratory journal 53: 2019.
- Wu X, Yang T, Zhou Q, Li S, Huang L: Additional use of a phosphodiesterase 5 inhibitor in patients with pulmonary hypertension secondary to chronic systolic heart failure: a meta-analysis. Eur J Heart Fail 16: 444-453, 2014.
- www.escardio.org/The-ESC/Press-Office/Press-releases/dapagliflozin-reduces-death-and-hospitalisation-in-patients-with-heart-failure, last accessed on 2019-09-03).
CARDIOVASC 2019; 18(6): 11-15











