Guideline changes and new therapeutic approaches are leading to changes in approaches to the treatment of severe asthma. In severe, refractory cases, biologics are now clearly preferred over steroids.
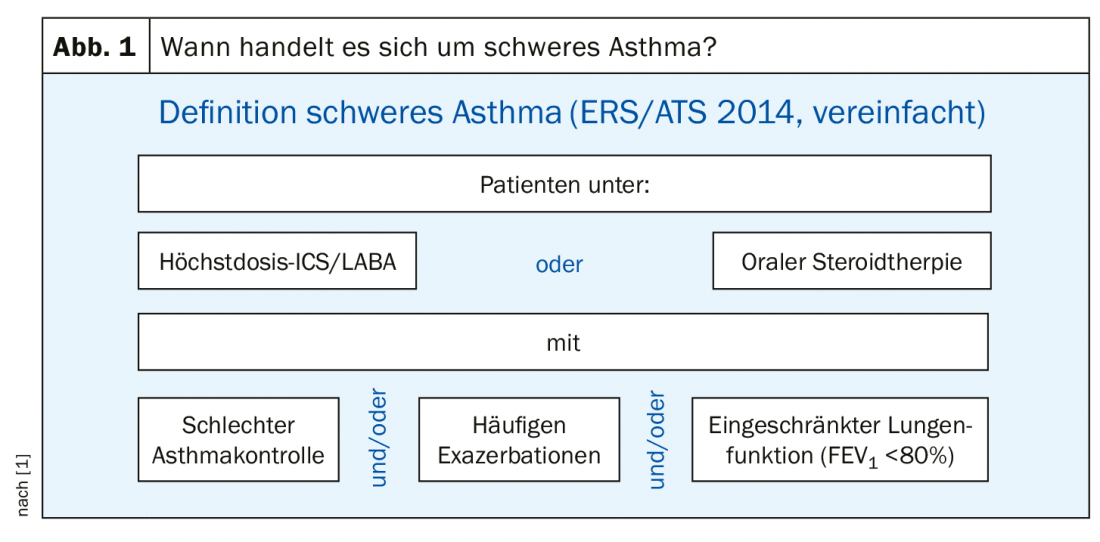
According to the guidelines of the European Respiratory Society (ERS) and the American Thoracic Society (ATS), the following constellation must occur in order to be considered a severe asthmatic: The patient receives therapy with an inhaled steroid and a betamimetic (ICS/LABA) on the one hand – and the steroid in maximum dose – or oral steroid therapy for at least six months per year. On the other hand, if the patient has either poor asthma control and/or frequent exacerbations and/or impaired lung function (FEV1 <80%) despite any of these therapies, then the patient is said to have severe asthma. In other words: Anyone who receives maximal or very high therapy but is not well controlled under it is a severe asthmatic. In Germany, this definition of severe asthma was adopted from the international ERS and ATS guidelines in 2014 (Fig. 1).
Severity levels as a grading scheme
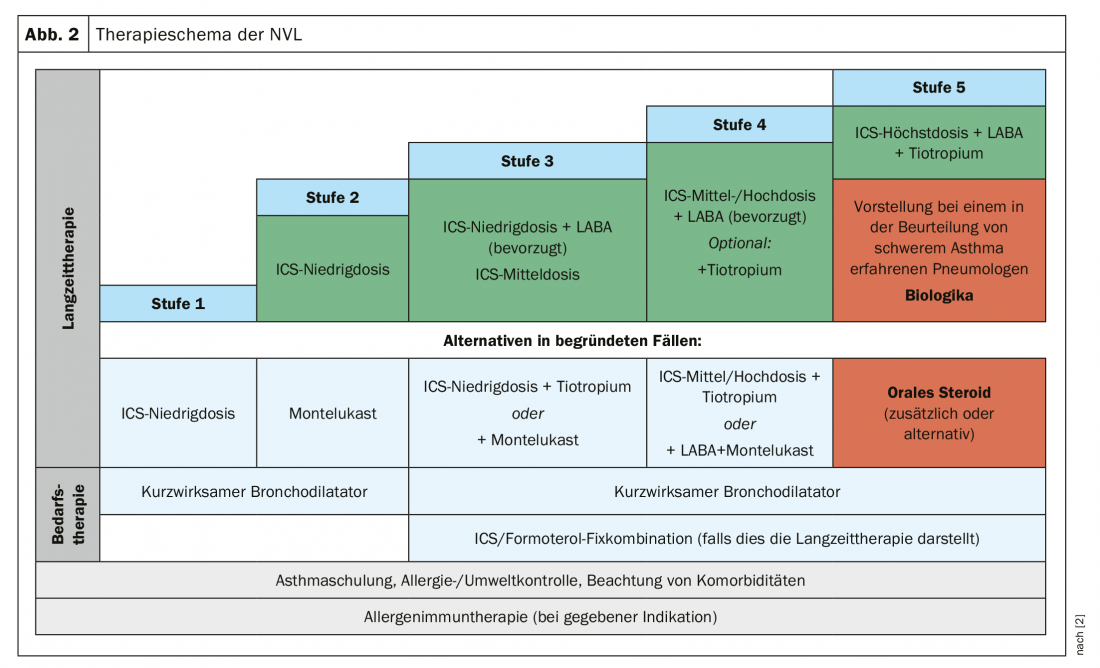
As of September 2018, our neighbors have a new National Health Care Guideline (NVL) that presents asthma severity levels as a grading scheme. In the first stage, the physician usually prescribes short-acting bronchodilators. This includes patients who have a very mild form of asthma. From level 2, we are dealing with patients who need the emergency inhaler at least twice a week. This is where you should start thinking about ICS therapy as a permanent therapy. “This is still the best horse in the stable,” says Prof. Dr. Marek Lommatzsch, Department of Pneumology, Rostock University Hospital. An alternative would be montelukast, such as in patients who have a fear of steroids. However, it is less effective – at least in adults.
If the ICS is not sufficient, one does not immediately go to the maximum dose, but combines it with a betamimetic. And if that is not enough either, a third inhalant can be added, namely tiotropium as the only approved anticholinergic. However, the real minor revolution in the current NVL was that for the first time the ICS/LABA fixed combination was recommended. Ten years ago it was still said that ICS and LABA should be given separately. Background: “It has been noticed that many patients get ICS and LABA separately, but then only take the LABA,” explains Prof. Lommatzsch. “And you know that LABAs are banned as monotherapy in asthma!”. With the fixed combination, this possibility is excluded.
Finally, in stage 5 with the severe asthma cases, an attempt should be made to install maximal inhaled therapy at least temporarily, over a period of at least three months, if the patient is not adequately controlled. That is, maximum dose ICS plus LABA plus LAMA (tiotropium) (Fig. 2).
But what is the effect of max-dose therapy exhaustion on biomarkers? Prof. Lommatzsch and his team in Rostock started a trial: A group of patients with uncontrolled asthma and medium ICS dose was packed on maximum dose for three months. The therapy had a decisive effect on the eosinophils, reducing them by about 50%. That eosinophils are massively reduced with oral or systemic steroids has been known for a long time, but it was not clear until now that ICS also have a significant effect on this biomarker. “This means that, on the one hand, we need to see the value of Eos in the context of medication, but on the other hand, we still know far too little about what the systemic effect of highest-dose ICS actually is.”
Physician must justify steroid use
But what about the patients who are on inhaled therapy to the maximum extent, can inhale properly, do everything the doctor wants them to do, but are still refractory to therapy? For such cases, the current NVL has the next small revolution ready: In these patients, biologics should be considered now at the latest. The only alternative would be steroids, but when using them, the physician must now justify why he prefers them to biologics.
This is because, according to Prof. Lommatzsch, asthmatics often have a love-hate relationship with systemic steroids. “Asthmatics love prednisolone. That’s almost a diagnostic criterion: If you ask an asthmatic how he finds prednisolone, he usually says: super. A COPD sufferer, on the other hand, says: It doesn’t help at all, only side effects. As an example of this “wonderful devil stuff” he cites a 56-year-old patient from his clinic who had been taking 80 mg of prednisolone orally every day since 1993 and 100 mg i.v. every two days and actually had no problems with her asthma under that. “She had obesity, diabetes, hypertension, osteoporosis, fractures, sleep disorders and depression, but wanted to see all the other doctors but me because she thought her asthma control was perfect after all.”
To avoid such cases in the future, biologics are needed as an alternative. To find the right biologic for a patient, phenotyping is first advisable. Prof. Lommatzsch lists three forms of asthma, which must first be identified:
- Transient asthma/wheezing appears in childhood and then disappears.
- Early-onset asthma begins in childhood and persists throughout life.
- Adult-onset asthma does not begin until adulthood.
- Similarly, biomarkers play an essential role in phenotyping and ultimately treatment decisions:
- Blood eosinophil concentration (cells/µl)
- Total IgE concentration in serum
- Prick test (typical allergens) Spec. IgE concentration in serum
- Exhaled NO (FeNO) in ppb (IGeL service)
Finding the right biologic
Since the approval of the anti-IL 4/13 biologic dupilumab in May 2019, pulmonologists have a total of five biologics available. Omalizumab (anti-IgE) has not only anti-allergic, but also anti-autoimmune and antiviral activity. A typical omalizumab patient has early-onset asthma, allergies, and high total IgE. In addition, there are the anti-IL-5 biologics mepolizumab, reslizumab, and benralizumab. The typical anti-IL-5 patient has adult-onset asthma has no allergies and marked blood eosinophilia. However, the overlap between the groups is quite high. For this reason, not only biomarkers and medical history are important in choosing the right biologic, but also comorbidities (Fig. 3) .
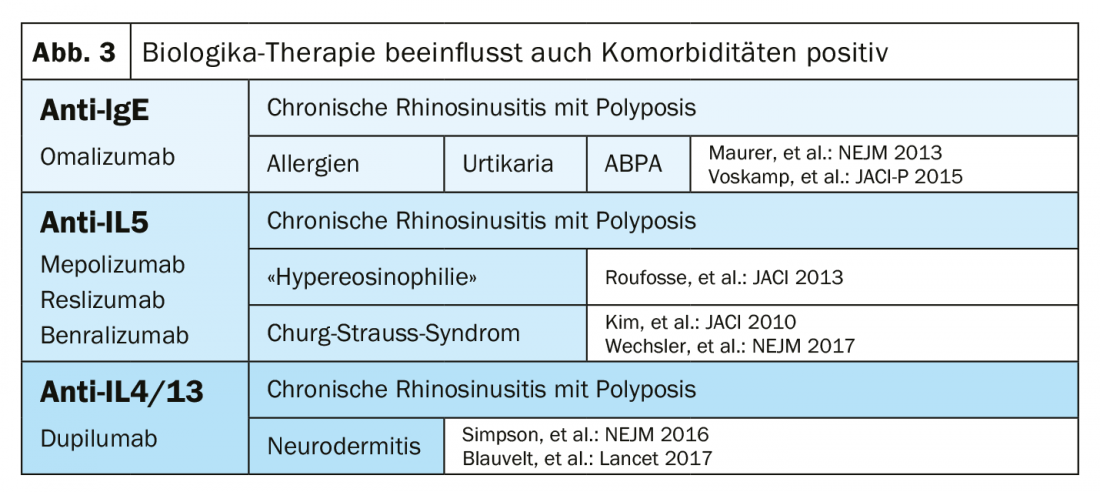
These three factors, phenotype, biomarkers, and comorbidities, the expert concluded, should always be carefully considered and weighed by pulmonologists, as they are crucial for choosing the right biologic.
Source: DGIM 2019, Wiesbaden (D)
Literature:
- Lommatzsch M, Virchow JC: Severe asthma: definition, diagnosis and treatment. Dtsch Arztebl Int 2014; 111: 847-855.
- BÄK, KBV, AWMF: Nationale Versorgungsleitlinie Asthma. Long version, 3rd edition. Version 1, 2018. www.leitlinien.de/mdb/downloads/nvl/asthma/asthma-3aufl-vers1-lang.pdf, last accessed 11/06/19.
InFo PNEUMOLOGY & ALLERGOLOGY 2019; 1(2): 18-20 (published 9/27/19, ahead of print).
HAUSARZT PRAXIS 2019; 14(10): 18-19