Depression is clustered in various brain disorders and presents a diagnostic challenge. Increased prevalence, partially specific etiopathogenetic mechanisms, and differential response to antidepressant treatments are characteristic in cerebrovascular insult (stroke), Parkinson’s disease, multiple sclerosis, epilepsy, and Alzheimer’s disease.
The following article discusses the various brain diseases in which depression is common.
Stroke
About one third of patients develop so-called post-stroke depression (PSD) after a stroke [1, 2]. Aphasia, anosognosia, alexithymia, and cognitive impairment may complicate clinical exploration and the use of standardized self-report scales. Observable behaviors such as facial expressions, tendency to cry, catastrophizing thoughts, etc. are helpful for diagnosis [1]. Table 1 provides an overview of the risk factors for PSD, such as the degree of physical impairment, severity of stroke, and cognitive impairment [1, 3].

An important cause of PSD is the psychosocial stress caused by the disruption to previous life and the resulting disability. Studies on whether specific locations of infarction lead to an increased risk of PSD are conflicting [1, 3]. Ischemia-specific effects are also discussed, although the underlying mechanisms are still unclear. A decrease in the monoamines serotonin, norepinephrine, and dopamine due to ischemia has been discussed [4]. Since the presence of depression also increases the risk of subsequent stroke by approximately 1.5-fold, there is likely a bidirectional relationship between the two conditions [3].
PSD responds well to antidepressants, although the current evidence is still rather weak. Antidepressant treatment may even favorably affect neurorehabilitation [4]. SSRIs are preferred because of their more favorable side effect profile. The best data are for fluoxetine and citalopram [3]. The current study situation rather argues against a frequently discussed antidepressant preventive treatment after stroke [3, 5]. To date, no evidence for the effectiveness of psychotherapies has been found.
Parkinson’s disease
Around 35-40% of patients with Parkinson’s disease develop depressive symptoms in the course of the disease. These may precede the development of motor symptoms. Anxiety disorders also occur more frequently. Due to overlapping symptoms such as hypomimia, motor slowing, fatigue, sleep disturbances, and weight loss, diagnosis is difficult, and the use of clinical symptom scales such as the Beck Depression Inventory (BDI) and the Hamilton Depression Scale (HAMD) is recommended [6, 7].
Reduced noradrenergic and serotonergic projections from the locus coeruleus and nucleus raphe may play a role in pathogenesis [8].
There are few good studies on the effectiveness of antidepressants. The efficacy of tricyclics (TCAs) is better established than that of SSRIs [6]. However, due to increased risk of falls due to sedation and anticholinergic effects, and thus the risk of worsening cognitive symptoms in the later course, SSRIs are to be preferred and the use of TCAs should be well weighed [3, 7]. A new placebo-controlled double-blind study showed good efficacy and tolerability of paroxetine and venlafaxine [9]. Although non-motor symptoms generally respond rather poorly to dopamine agonists [8], pramipexole showed good effects on mild to moderate depression [10]. Data on the efficacy of psychotherapy are insufficient, but there is evidence of an antidepressant effect of cognitive behavioral therapy (CBT) [3].
Multiple Sclerosis (MS)
The lifetime prevalence of depressive disorders is estimated to be as high as 50% in people with MS. Up to a quarter of people with MS develop suicidal ideation, with depression being an important predictor. The diagnosis of depression in MS is also complicated due to overlapping symptoms such as fatigue, sleep disturbances, appetite disturbances, and memory and concentration problems, and can be improved by self-rating scales such as the BDI [3, 11].
A psychosocial factor is the stress caused by the disabling disease, although no association was found with the degree of disability and the duration of the disease. Rather, coping strategies matter, with an increased risk of emotion-focused versus action-focused coping [11]. It is assumed that the global brain damage of the disease leads to a generally increased vulnerability of the emotional systems. There is also evidence of dysregulation of the stress axis between the hypothalamus, pituitary, and adrenal cortex (HPA axis). Studies of the dependence of depressive symptoms on lesion distribution have been hard pressed to find consistent patterns. In contrast, associations were found between depression and higher left frontal lesion volume and left temporal regional brain atrophy [3, 11].
Depression may occur as a side effect of drug treatment with interferon beta. Main risk factor is depressive episodes before treatment. Depressive symptoms respond well to antidepressant therapy, thus treatment usually does not need to be discontinued [12].
There is still little evidence on pharmacological treatment [13], but the use of antidepressants – rather SSRIs due to their more favorable side effect profile – is highly recommended [14]. CBT focusing on coping strategies as well as mindfulness stress reduction have been shown to be effective [11, 14].
Epilepsy
The risk of developing depression is increased by approximately 1.4-fold in epilepsy, thus the prevalence rate is higher in stroke, Parkinson’s disease, and MS. However, the risk of suicide is .increased by up to 10-fold in epilepsy, depending on the study, making the detection and treatment of depression important in suicide prevention [15, 16]. Before or after a seizure, acute mood deterioration may occur for hours to days, which should be distinguished from clinical depression [15].
Etiopathogenetically, a multifactorial diathesis-stress model is assumed, with epilepsy causing a state of chronic stress. Learned helplessness and the unpredictability of seizures play a role as psychological factors [3, 15]. Studies of the HPA axis have shown that, as in depression, it is also dysregulated in epilepsy-particularly temporal lobe epilepsy [3, 17]. In addition, early childhood deprivation in animal models leads to both an increased prevalence of depressive behavior and increased epileptogenesis. Similar changes can also be found in the metabolism of glutamate and GABA in animal models of both diseases [17].
SSRIs are effective, although again there are still too few good placebo-controlled studies. However, because of the increased risk of suicide in epilepsy with comorbid depression, the use of SSRIs is highly recommended [15]. Epilepsy-specific CT programs and acceptance and commitment therapy (ACT) have also been shown to be effective, even demonstrating a reduction in seizure frequency [3, 15]
Antiepileptic drugs may have an effect on psychiatric symptoms, especially mood (Table 2) . Topiramate, vigabatrin, levetiracetam, tiagabine, and zonisamide are more likely to be associated with negative effects, while gabapentin, pregabalin, lacosanide, valproate, carbamazepine, and lamotrigine are associated with positive effects [18]. In cases of comorbid depression, switching to a mood-stabilizing anticonvulsant with a positive effect is recommended whenever possible [19].
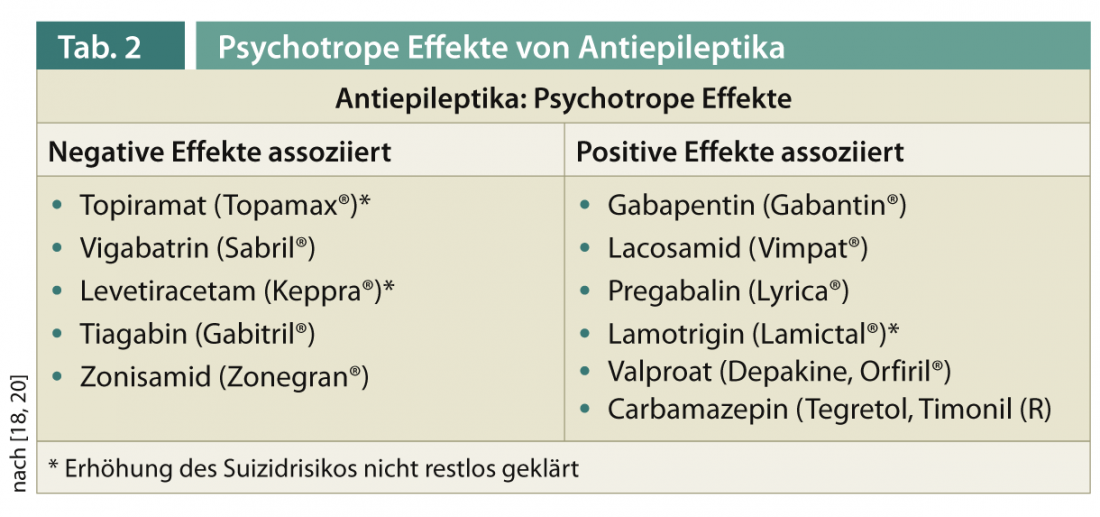
An earlier warning by the U.S. Food and Drug Administration regarding a possible increased risk of suicide from antiepileptic therapy has since been qualified due to unconvincing data [20].
Dementia, especially Alzheimer’s disease
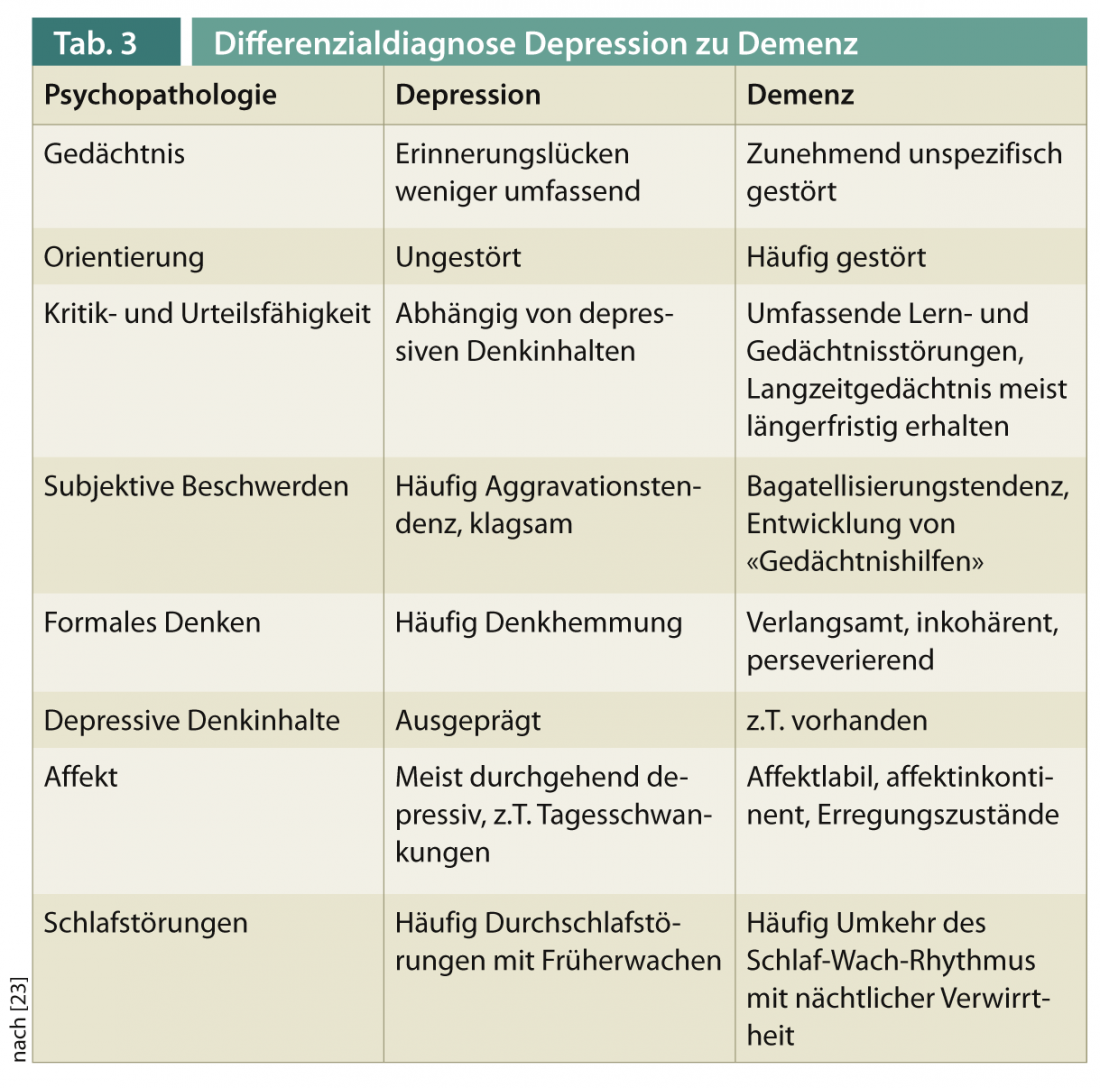
The differential diagnosis between dementia with secondary depressive symptoms and depression with secondary cognitive impairment is a clinical challenge in patients of advanced age. Table 3 provides an overview of psychopathological differentiation features.
In AD, neuropsychiatric disorders (NPS) are often encountered, most commonly apathy and depressive symptoms. Prodromally, NPS also play a role: for example, patients with mild cognitive impairment and NPS have a much higher risk of later onset of AD than those without NPS [21]. NPS in the elderly are often the first signs of dementia development, most pronounced in frontotemporal dementia [22].
Depression, on the other hand, is a risk factor for dementia; for example, the number of depressive episodes correlates with the later onset of Alzheimer’s disease [23].
In the future, better pathophysiologically based concepts are hoped for differentiation. An overlapping pathophysiology of noradrenergic systems is conceivable, but not yet clearly defined, e.g., in Alzheimer’s dementia (as in Parkinson’s disease) a reduction of noradrenergic neurons in the locus coeruleus is found, which may also play a role in the development of affective symptoms [23, 24].
Differentiating depression in older age from depressive symptoms as NPS of AD is particularly relevant because there is limited evidence for the efficacy of antidepressant medication in AD [21, 25], whereas the efficacy of antidepressants and psychotherapeutic treatment of depression in older age is well established [23]. Because of better tolerability, SSRIs are preferred for depressive symptoms of AD. NPS in AD respond moderately to antipsychotics. However, their use should be carefully considered due to potentially serious side effects [21].
Christian Imboden, MD
Literature:
- Carota A, Bogousslavsky J: Mood disorders after stroke. Front Neurol Neurosci 2012; 30: 70-74.
- Kouwenhoven SE, Kirkevold M, Engedal K, Kim HS: Depression in acute stroke: prevalence, dominant symptoms and associated factors. A systematic literature review. Disabil Rehabil 2011; 33(7): 539-556.
- Piber D, Hinkelmann K, Gold SM, Heesen C, Spitzer C, Endres M, et al: Depression and neurological disorders. Neurologist 2012; 83(11): 1423-1433.
- Loubinoux I, Kronenberg G, Endres M, Schumann-Bard P, Freret T, Filipkowski RK, et al: Post-stroke depression: mechanisms, translation and therapy. J Cell Mol Med 2012; 16(9): 1961-1969.
- Hackett ML, Anderson CS, House AO, Xia J: Interventions for Treating Depression After Stroke. Stroke 2009 May 14.
- 6. Tan LC: Mood disorders in Parkinson’s disease. Parkinsonism Relat Disord 2012; 18 Suppl 1: S74-S76.
- Schwarz J, Odin P, Buhmann C, Csoti I, Jost W, Wullner U, et al: Depression in Parkinson’s disease. J Neurol 2011; 258 (Suppl 2): S336-S338.
- Preacher RD, Matheus FC, Schwarzbold ML, Lima MM, Vital MA: Anxiety in Parkinson’s disease: a critical review of experimental and clinical studies. Neuropharmacology 2012; 62(1): 115-124.
- Richard IH, McDermott MP, Kurlan R, Lyness JM, Como PG, Pearson N, et al: A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology 2012; 78(16): 1229-1236.
- Barone P, Poewe W, Albrecht S, Debieuvre C, Massey D, Rascol O, et al: Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9(6): 573-580.
- Feinstein A: Multiple sclerosis and depression. Mult Scler 2011; 17(11): 1276-1281.
- Feinstein A, O’Connor P, Feinstein K: Multiple sclerosis, interferon beta-1b and depression A prospective investigation. J Neurol 2002; 249(7): 815-820.
- Koch MW, Glazenborg A, Uyttenboogaart M, Mostert J, De Keyser J: Pharmacologic treatment of depression in multiple sclerosis. Cochrane Database Syst Rev 2011 Feb 16;(2)(2):CD007295.
- Skokou M, Soubasi E, Gourzis P: Depression in multiple sclerosis: a review of assessment and treatment approaches in adult and pediatric populations. ISRN Neurol 2012; 2012: 427102.
- Hoppe C, Elger CE: Depression in epilepsy: a critical review from a clinical perspective. Nat Rev Neurol 2011; 7(8): 462-472.
- Bagary M: Epilepsy, antiepileptic drugs and suicidality. Curr Opin Neurol 2011; 24(2): 177-182.
- Kanner AM: Depression and epilepsy: A bidirectional relation? Epilepsia 2011; 52 Suppl 1: 21-27.
- Piedad J, Rickards H, Besag FM, Cavanna AE: Beneficial and adverse psychotropic effects of antiepileptic drugs in patients with epilepsy: a summary of prevalence, underlying mechanisms and data limitations. CNS Drugs 2012; 26(4): 319-335.
- Pauli E, Stefan H: Emotional-affective disturbances in epilepsies. Neurologist 2009; 80(6): 729-744.
- Fountoulakis KN, Gonda X, Samara M, Siapera M, Karavelas V, Ristic DI, et al: Antiepileptic drugs and suicidality. J Psychopharmacol 2012; 26(11): 1401-1407.
- Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al: Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement 2011; 7(5): 532-539.
- Taragano FE, Allegri RF, Krupitzki H, Sarasola DR, Serrano CM, Lon L, et al: Mild behavioral impairment and risk of dementia: a prospective cohort study of 358 patients. J Clin Psychiatry 2009; 70(4): 584-592.
- Hatzinger M: Affective disorders in old age. Swiss Archives of Neurology and Psychiatry 2011; 162(5): 179-189.
- Szot P: Common factors among Alzheimer’s disease, Parkinson’s disease, and epilepsy: possible role of the noradrenergic nervous system. Epilepsia 2012; 53 Suppl 1: 61-66.
- Even C, Weintraub D: Case for and against specificity of depression in Alzheimer’s disease. Psychiatry Clin Neurosci 2010; 64(4): 358-366.
InFo NEUROLOGY & PSYCHIATRY 2013; 11(1): 9-12.