Many patients with psoriatic arthritis (PsA) suffer from chronic symptoms and need permanent relief from their symptoms. Two pooled analyses based on data from the PALACE trial program show that efficacy and safety of the oral PDE4 inhibitor in PsA are sustained over a treatment period of up to 260 weeks, with improvements across multiple disease domains.
The results of the pooled analyses underscore the value of apremilast as an effective and tolerable second-line treatment for active psoriatic arthritis (PsA). The use of apremilast (Otezla®) is recommended in European guidelines for moderate to severe psoriasis and coexisting PsA, particularly in patients who are not suitable for biologic treatment and who have had an inadequate response to a csDMARD [1]. Apremilast therapy showed sustained improvements in long-term follow-up, including pressure painful and swollen joints, as well as dactylitis, enthesitis, and in PASI-75. Even after five years, there were no clinically relevant changes in the safety profile – rates of adverse events remained low.
Pooled analyses based on multiple Phase III studies
The PALACE 1-3 randomized placebo-controlled trials evaluated the efficacy and safety of apremilast in patients with active PsA over the long-term course of up to 5 years [2]. The inclusion criteria at baseline were the presence of active PsA for ≥ 6 months and three or more swollen and pressure-painful joints despite previous treatment with csDMARDs and/or biologics [2,3]. A total of 1493 patients were randomized to apremilast 30 mg twice daily (n=497), apremilast 20 mg twice daily (n=500), or placebo (n=496) [3]. A 24-week placebo-controlled period was followed by a 28-week extension of blinded active treatment and then an open-label treatment phase lasting 4 years [2]. More than 40% of patients continued apremilast treatment for a total of 260 weeks in the long-term extension studies. No new security problems were encountered in the process.
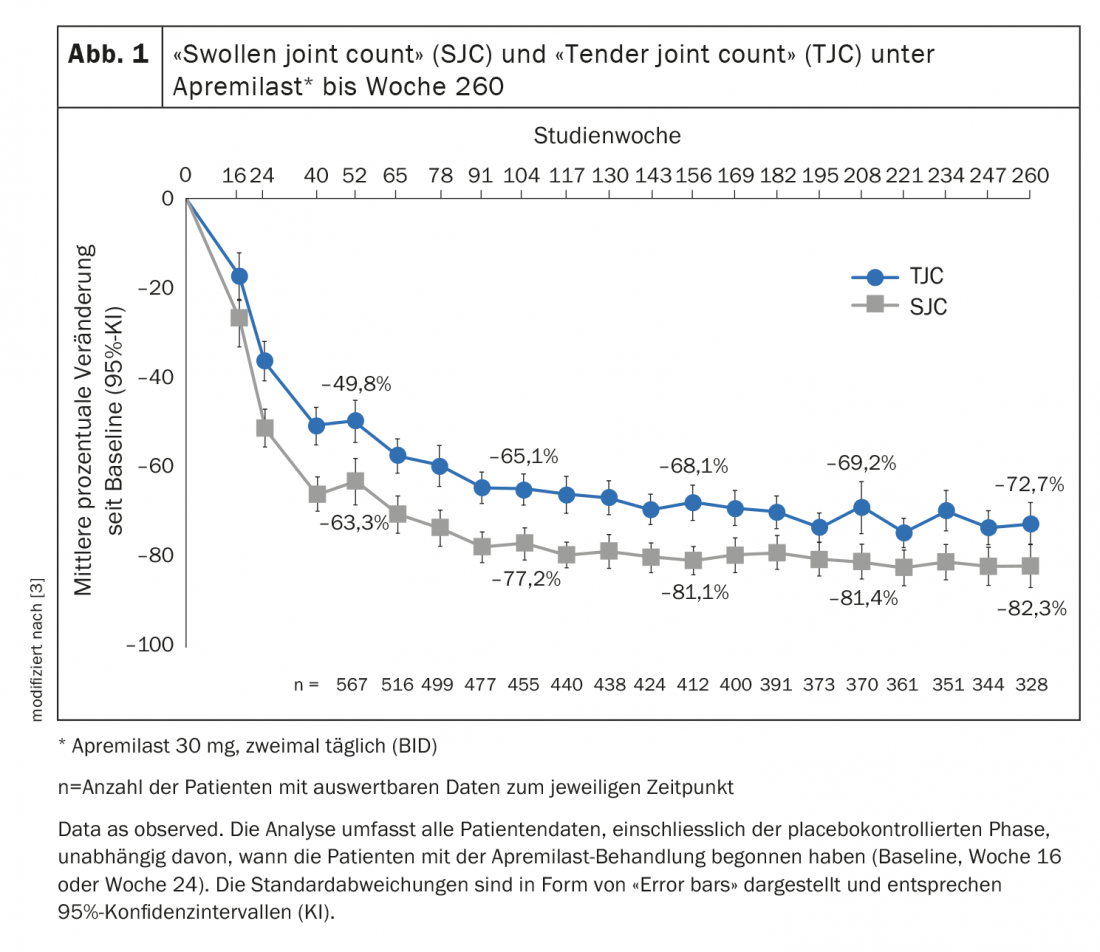
Sustained high ACR20 response rates and significant reduction in SJC/TJC.
Of patients receiving apremilast 30 mg twice daily, 55.3% achieved at least a 20% improvement in response at week 52 according to American College of Rheumatology criteria (ACR 20) [3]. Among those who continued apremilast treatment, the ACR20 response rate at week 260 was 67.2%. Regarding ACR50 and ACR70, the response rates were 44.4% and 27.4%, respectively. The number of pressure painful and swollen joints had decreased significantly after one year of treatment. This trend continued throughout the rest of the treatment period. Mean percent changes in the number of swollen or pressure-painful joints through week 260 are shown in Figure 1. In the “Swollen joint count” (SJC) and the “Tender joint count” (TJC) , there was an improvement of 63.3% and 49.8%, respectively, in week 52. With continued treatment, values of 82.3% and 72.7% were achieved at week 260, respectively.
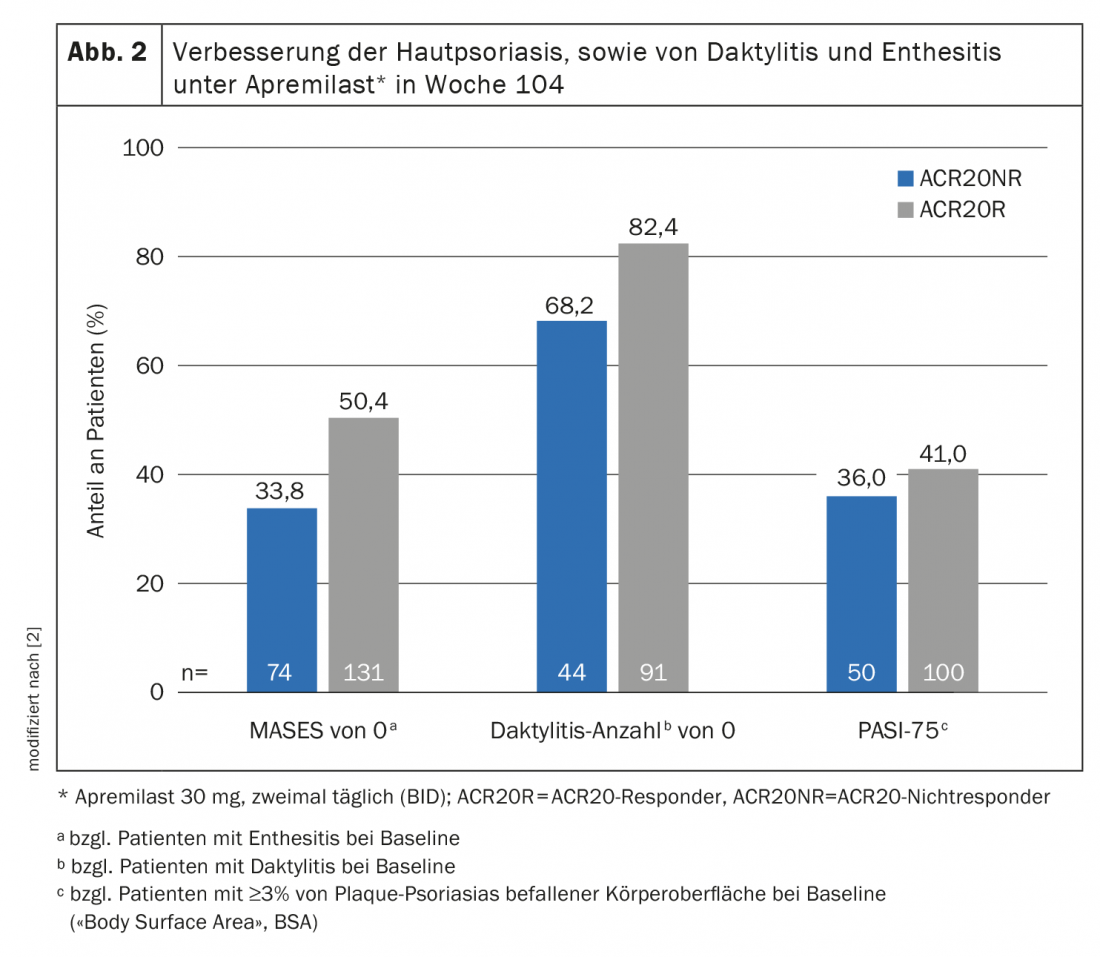
Improvement of skin psoriasis and dactylitis and enthesitis.
Of those patients with ≥3% body surface area (“BSA”) affected by plaque psoriasis, 43.6% had a PASI-75 response at week 260. In the subpopulation of study participants who had enthesitis and dactylitis at baseline, 62.4% achieved a Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) of 0 and 80.9% achieved a dactylitis score of 0 [3]. Overall, the efficacy and safety profile of apremilast in PsA proved to be persistently beneficial over a 5-year treatment period. The fact that one-third of those treated with apremilast who did not achieve an ACR20 response continued study participation until week 260 suggests that ACR response should not be used exclusively when assessing PsA disease activity [3]. This observation was the starting point of the pooled analysis by Mease et al. published in 2021, which demonstrated that ACR20 non-responders randomized to apremilast 30 mg twice weekly at baseline and continuing this treatment achieved clinically meaningful improvements at week 104 (Fig. 2) [2]. Thus, 33.4% achieved a MASES of 0 and 68.2% achieved a dactylitis count of 0. The PASI-75 response rate was 36% in this patient subpopulation. Moreover, the improvements in SJC and TJC also continued through week 104. This indicates that relief of symptoms not mapped by an ACR response are also clinically relevant for PsA patients. Furthermore, the study authors noted that treatment with apremilast should be continued for at least 24 weeks, as the results of the pooled analyses suggest that the efficacy of this immunomodulatory agent reaches a plateau only after this period, with subsequent sustained improvements in several disease domains [2].
Literature:
- Nast A, et al: German S3 guideline on the therapy of psoriasis vulgaris adapted from EuroGuiDerm – Part 2: Therapy monitoring, special clinical situations and comorbidity. JDDG 2021; 19(7): 1092-1117.
- Mease PJ, et al: Articular and Extra-Articular Benefits in ACR20 Non-responders at Week 104 Treated With Apremilast: Pooled Analysis of Three Randomized Controlled Trials. Rheumatol Ther 2021; 8(4): 1677-1691.
- Kavanaugh A, et al: Long-term experience with apremilast in patients with psoriatic arthritis: 5-year results from a PALACE 1-3 pooled analysis. Arthritis Res Ther 2019; 21, 118. https://doi.org/10.1186/s13075-019-1901-3
DERMATOLOGIE PRAXIS 2022; 32(2): 32-33











