Type 2 diabetes develops insidiously over years and is usually diagnosed in middle to older adulthood – often incidentally during routine checkups. Adequate glycemic control is very important to prevent acute complications and long-term sequelae. If individual target levels are not achieved with oral antidiabetics and/or GLP-1 receptor agonists, insulin treatment should be initiated. Basal insulin is often sufficient at first; nowadays, long-acting insulin analogues are mostly used for this purpose.
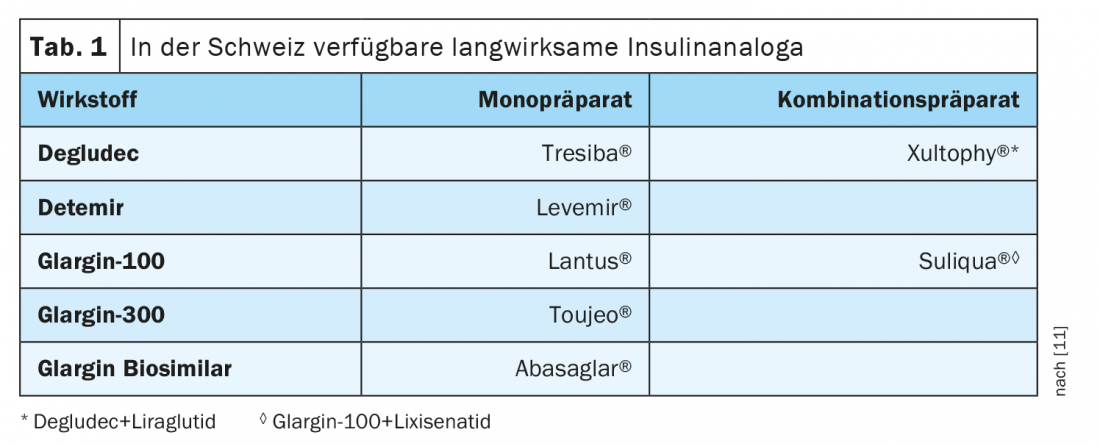
Pathophysiologically, in type 2 diabetes, insufficient insulin production or impaired insulin action results in inadequate uptake of glucose from the blood, resulting in elevated blood glucose levels. This is because the hormone insulin, produced by the beta cells of the pancreas, ensures that the body’s cells can absorb and process glucose. Therapy for type 2 diabetes aims to maintain blood glucose levels at the adequate level. Initially, lifestyle changes may be sufficient, with many patients requiring complementary drug treatment. As the disease progresses, insulin therapy may also become necessary. Basal insulin covers the basic insulin requirement regardless of the meal, even at night, and is injected at least once a day or more often, as appropriate. The long-acting basal insulin analogs (Table 1) have gained acceptance over NPH (neutral protamine hagedorn) insulin. In addition to a reduction in nocturnal hypoglycemia, another advantage is the simpler handling in the form of a clear solution, whereas the application of NPH insulin requires a prior suspension of the insulin.
Check indication for insulin therapy individually
Nowadays, the treatment strategies for type 2 diabetes are determined individually and with the involvement of the patient, explains Prof. Martin Pfohl, MD, Chief Physician, Bethesda Hospital Duisburg [1]. If HbA1c remains above the target range despite lifestyle changes (healthy diet and increased physical activity), antidiabetic drug treatment is recommended unless contraindications exist [2]. The German National Health Care Guideline Diabetes mellitus Type 2 recommends a target HbA1c corridor between 48 and 69 mmol/mol (6.5-8.5%) as a guideline value, although this may vary individually taking into account various patient characteristics such as age, comorbidities, hypoglycemia risk, or weight gain [3].
In most cases, oral therapy starts with metformin. If this does not achieve the HbA1c targets, one moves to combination therapy, which may include sulfonylureas, DPP-4 inhibitors, SGLT-2 inhibitors (SGLT2i), and/or GLP-1 receptor agonists (GLP-1-RA). If HbA1c is above the individual HbA1c target despite medication with oral antidiabetic agents and/or with an injectable GLP-1 analogue, and especially in cases of metabolic derailment (HbA1c value >10%), insulin administration is advised [2]. In the majority of type 2 diabetics, insulin dependence develops only after several years of diabetes, when absolute insulin deficiency occurs as a result of a beta cell defect. Whether initial insulin treatment is necessary in patients with type 2 diabetes must also be clarified on an individual basis – in the analysis of a Swedish working group on a cohort of 8980 persons with newly diagnosed diabetes, the proportion of type 2 diabetics with severe endogenous insulin deficiency (“severe insulin deficient diabetes”) was about 17.5% [4].
Temporary insulin treatment may also be necessary in the context of an infection or a pending operation, or other situations that lead to a deterioration of insulin action or an increased need for insulin [3].
Basal assisted oral therapy
Basal-assisted oral therapy (BOT) is well suited as the first form of insulin therapy for most type 2 diabetics. A fixed dose of basal insulin is usually administered in the evening. It was shown that the combination with metformin achieved similar glycemic control compared with insulin monotherapy, but body weight was more favorably affected [5,6]. Detemir (Levemir®) or glargine-100 (Lantus®) are recommended as a starting point, and in terms of titration regimen, a start with 10 E (more allowed in overweight patients) with an increase of 2 E every 2 days until fasting blood glucose <is 8 mmol/l, ideally 6-7 mmol/l [7,8]. (Caution: Do not increase basal insulin dose of about one-third of body weight further, but add bolus insulin) [7]. The ultra-long-acting Tresiba® may be prescribed if morning hyperglycemia occurs despite dosing with detemir or glargine-100, respectively, if blood glucose rises overnight or if the patient does not inject on his or her own before bedtime (Tresiba® can be injected at any time during the day).
Which patients benefit from ultralong-acting basal insulins?
Insulin glargine-300 and insulin degludec are currently the two insulin analogues with the longest duration of action and the lowest hypoglycemia risks (box). The older the type 2 diabetics, the more they tend to use these very long-acting second-generation basal insulins, explains Prof. Pohl. This goes back to the SENIOR study [17]. This examined how titration works in elderly patients and the incidence of hypoglycemia. Here, glargine-300 was found to be effective in older people (≥65 years) with a longer duration of diabetes, with lower rates of documented symptomatic hypoglycemia compared with glargine-100, particularly in patients ≥75 years. These results are consistent with data from a post-hoc meta-analysis of the EDITION 1, 2, and 3 trials in adults aged ≥65 years.
| Ultralong-acting insulin analogues
In the course of type 2 diabetes, insulin therapy is often necessary, especially in cases of prolonged diabetes and/or severe renal insufficiency [11]. Studies show that the newer ultralong-acting basal insulin analogues, such as insulin degludec (Tresiba®) or insulin glargine 300 (Toujeo®), result in significantly less hypoglycemia than the existing basal insulins [12,13]. The DELIVER high-risk study demonstrated that in type 2 diabetic patients at high risk of hypoglycemia, glargine-300 – a second-generation basal insulin analog – was associated with a significantly lower risk of hypoglycemia than first-generation basal insulin analogs one year after switching from a first-generation basal insulin, with comparable glycemic control [16]. Fixed combinations with GLP-1-RA combine several advantages. [14,15]Insulin degludec combined with the GLP-1 RA liraglutide resulted in better glycemic control and fewer hypoglycemias compared with insulin glargine 100 . The DEVOTE study showed that there is no higher cardiovascular risk with degludec compared to glargine [6]. |
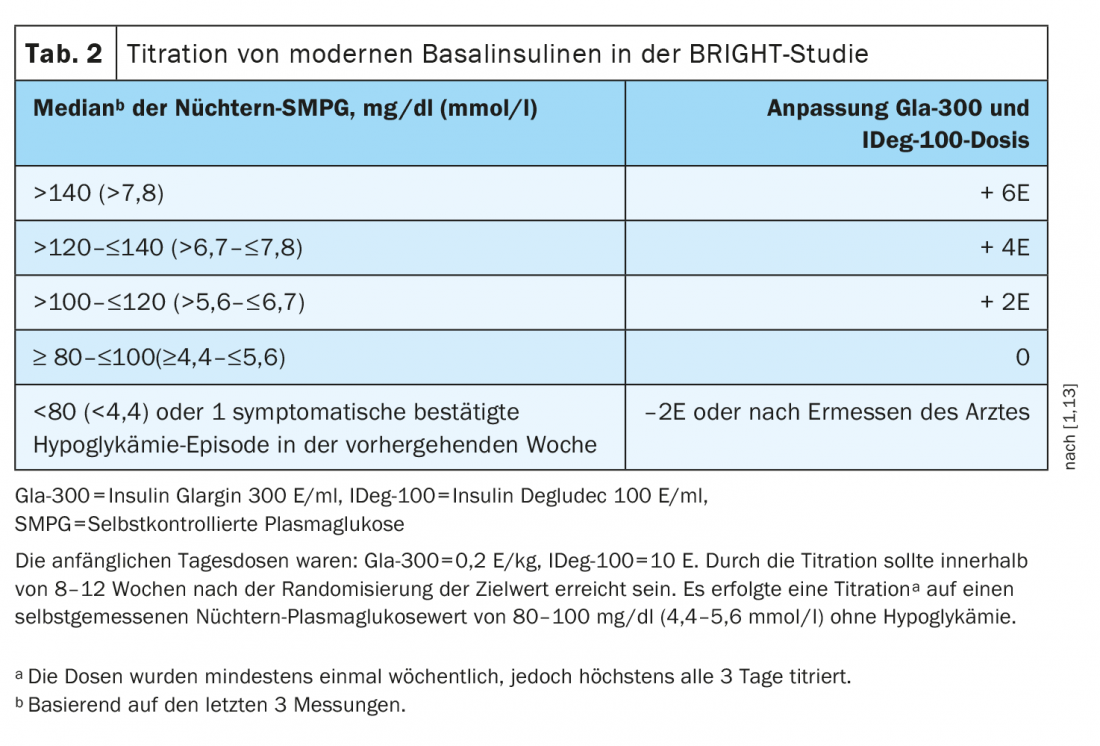
In patients with impaired renal function, glargine-300 was superior to insulin degludec in the BRIGHT study. This is shown by a subgroup analysis in which a greater HbA1c reduction was achieved with glargine-300 than with insulin degludec in type 2 diabetics withrenal insufficiency[9]. BRIGHT was a 24-week, multicenter, open-label, randomized, active-controlled, two-arm, parallel-group study in insulin-naïve uncontrolled type 2 diabetes. Participants were randomized 1:1 to glargine-300 (n=466) or insulin degludec-100 (n=463) and stratified for analysis based on estimated glomerular filtration rate (eGFR). Glargine-300 and insulin degludec-100 were administered once daily between 18 and 20 Clock administered by the patients themselves (Tab.2). In particular, in a subgroup of patients with an eGFR <60 ml/min/1.73 m2, glargine-300 was associated with significantly higher mean HbA1c reductions from baseline to week 24 (8.58% to 6.94%) compared with insulin degludec-100 (8.30% to 7.28%) [9]. Self-measured glucose profiles were also found to be better in this subgroup under glargine-300, whereas in type 2 diabetics with unrestricted renal function the values were approximately the same. Prof. Pfohl said that it was not known exactly why this was the case.
If BOT is not sufficient: fixed combination with GLP-1-RA
Basal insulins can also be combined with GLP-1 receptor agonists in a pen. Especially for very overweight type 2 diabetics who already require insulin therapy, the combination of insulin and incretin mimetics makes sense, as it can prevent rapid weight gain. Xultophy® is a fixed combination of GLP-1-RA (liraglutide) and insulin degludec [8]. Suliqua® is a combination preparation of GLP-1-RA and insulin glargine-100 [8]. Dosing is individualized for both fixed combinations and titrated according to the patient’s insulin needs. The application is carried out once a day.
In the LixiLan-L trial, significantly more patients achieved HbA1c <7% without hypoglycemia or weight gain under the fixed combination of GLP-1-RA and insulin glargine than under insulin glargine 100 E/ml (p<0.0001) [10].
Kongess: Diabetology without borders
Literature:
- Pfohl M: Importance of the titration phase in type II diabetes mellitus with basal insulin. Diabetology Limitless, 04.02.2022
- “Oral antidiabetic agents and GLP-1 analogues,” Swiss Med Forum 2019; 19(1920): 339-340.
- National Health Care Guideline Diabetes 2021, www.leitlinien.de/themen/diabetes/2-auflage/kapitel-2#abb6 (last accessed Feb. 24, 2022).
- Ahlqvist E, et al: Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018; 6(5): 361-369.
- Institute for Quality and Efficiency in Health Care. Long-acting insulin analogues for the treatment of type 2 diabetes mellitus; final report; version 1.1; commission A05-03 [online]. 02/26/2009 www.iqwig.de (last accessed 02/24/2022)
- Physician’s Manual. The treatment pathway to the disease management program. Diabetes mellitus type 2. November 2020, as of www.arztnoe.at (last accessed Feb. 24, 2022).
- Kohler S, Beise U, Huber F: Diabetes mellitus, medix Guideline, www.medix.ch (last accessed Feb. 24, 2022).
- Swissmedic: Medicinal product information, www.swissmedicinfo.ch (last accessed 24.02.2022).
- Haluzík M, et al: Differential glycaemic control with basal insulin glargine 300 U/mL versus degludec 100 U/mL according to kidney function in type 2 diabetes: A subanalysis from the BRIGHT trial. Diabetes Obes Metab 2020; 22(8): 1369-1377.
- Aroda VR; LixiLan-L Trial Investigators: Efficacy and Safety of LixiLan, a Titratable Fixed-Ratio Combination of Insulin Glargine Plus Lixisenatide in Type 2 Diabetes Inadequately Controlled on Basal Insulin and Metformin: The LixiLan-L Randomized Trial. Diabetes Care. 2016 Nov;39(11): 1972-1980.
- Schneider L, Lehmann R: Swiss Diabetes Guide. Swiss Med Forum 2021; 21(1516): 251-256.
- Marso SP, et al: Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med 2017; 377(8): 723-732.
- Rosenstock J, et al: More Similarities Than Differences Testing Insulin Glargine 300 Units/mL Versus Insulin Degludec 100 Units/mL in Insulin-Naive Type 2 Diabetes: The Randomized Head-to-Head BRIGHT Trial. Diabetes Care 2018; 41(10): 2147-2154.
- Lingvay I, et al: Effect of Insulin Glargine Up-titration vs Insulin Degludec/Liraglutide on Glycated Hemoglobin Levels in Patients With Uncontrolled Type 2 Diabetes: The DUAL V Randomized Clinical Trial. JAMA 2016; 315(9): 898-907.
- Billings LK, et al: Efficacy and Safety of IDegLira Versus Basal-Bolus Insulin Therapy in Patients With Type 2 Diabetes Uncontrolled on Metformin and Basal Insulin: The DUAL VII Randomized Clinical Trial. Diabetes Care 2018; 41(5): 1009-1016.
- Pfohl M, et al: Clinical Diabetology. Type 2 diabetic patients at high risk of hypoglycemia on first-generation basal insulin analogues had a lower risk of hypoglycemia after switching to insulin glargine 300 E/ml (Gla-300) (DELIVER High-Risk Study). Diabetology and Metabolism 2021; 16(S 01): S13
- Ritzel R, al: A randomized controlled trial comparing efficacy and safety of insulin glargine 300 units/mL versus 100 units/mL in older people with type 2 diabetes: Results from the SENIOR Study. Diabetes Care 2018; 1672-1680.
HAUSARZT PRAXIS 2022; 17(3): 30-31
CARDIOVASC 2022; 21(2): 30-31











