Both infectious and noninfectious granulomatous dermatoses are very heterogeneous clinical pictures. Treatment is based on pathogenesis and is primarily anti-inflammatory, with corticosteroids still playing an important role. A new therapeutic target is the JAK-STAT pathway, and there are some recent study findings on this. The methodology of RNA sequencing used for this purpose provides insight into pathophysiologically relevant genetic features and reveals interesting correlations.
“Granulomatous dermatoses are an extremely diverse group of diseases,” explains Prof. Dr. med. Mario Fabri of the University Hospital of Cologne at this year’s meeting of the German Dermatological Society (DDG) [1]. With regard to pathomechanisms, progress has been made by modern high-throughput methods, but there are still many open questions. It is expected that further “omics” data will help to improve pathophysiological understanding. In addition to metabolomics and proteomics, “spatial transcriptions” (gene expression that can be localized on histological sections) are also used in the field of genomics. Regarding therapeutic options, recent studies have identified the JAK-STAT pathway as a potential therapeutic target. “JAK-STAT could be established as a new therapeutic target,” Prof. Fabri said.
Defense mechanism against infectious and non-infectious influences
Benign connective tissue nodules, called granulomas, can occur in all organs. “The granuolome, by definition, is a nodular collection of macrophages,” the speaker explained [1]. According to the histomorphologic structure of the granulomas, tuberculoid, sarcoid, collagenolytic, or palisade granulomas are classified, Pseudotuberculosis-type granulomas and foreign body granulomas are distinguished [2]. According to current knowledge, it is assumed that the formation of a granuloma represents a specific defense mechanism of the organism, whereby a central stimulus, which can be of infectious (mycobacteria) or non-infectious (foreign bodies) origin, results in the activation of skin antigen-presenting cells, which subsequently attract both granulocytes and lymphocytes to the tissue [2]. These inflammatory cells usually accumulate in a ring around the antigen-presenting cells and form the characteristic histological picture of the granuloma. Activation of tissue-derived macrophages phagocytose infectious agents or inert foreign bodies in an attempt to isolate and thus contain potentially virulent pathogens. It is therefore a kind of protective wall around material that cannot be eliminated, explains Prof. Fabri.
RNA sequencing to explore pathomechanisms.
“Molecular mechanisms have been shown primarily in animal models,” the expert said. In recent years, progress has also been made in research in humans. Analyses with modern high-throughput methods lead(ed) to a new understanding. Leprosy is an example of an infectious granulomatosis in which the pathogenesis has been studied by such modern methods. “Infectious granulomatoses can be caused by bacteria, parasites, fungi, as well as viruses,” the speaker explained. Leprosy is caused by the pathogen Mycobacterium leprae. In an article by Inkeles et al. [3] reports a study in which RNA sequencing was performed and analyzed using complex mathematical models. For example, researchers have found that there is a network of dendritic cells in tuberculoid leprosy in which matrix metalloproteinase 12 (MMP12) likely plays a major pathogenetic role.
Sarcoidosis and granuloma anulare: Corticosteroids still first choice
Primary therapy for sarcoidosis consists of corticosteroids, which may be administered topically or intralesionally; systemic administration may also be necessary [4,5]. Alternatively, hydroxychloroquine, methotrexate (MTX), or dapsone may be used for anti-inflammatory therapy. In addition, TNF-alpha blockers (off-label) have been shown to improve disease. However, latent tuberculosis must be ruled out before treatment is attempted (quantiferon test) [5].
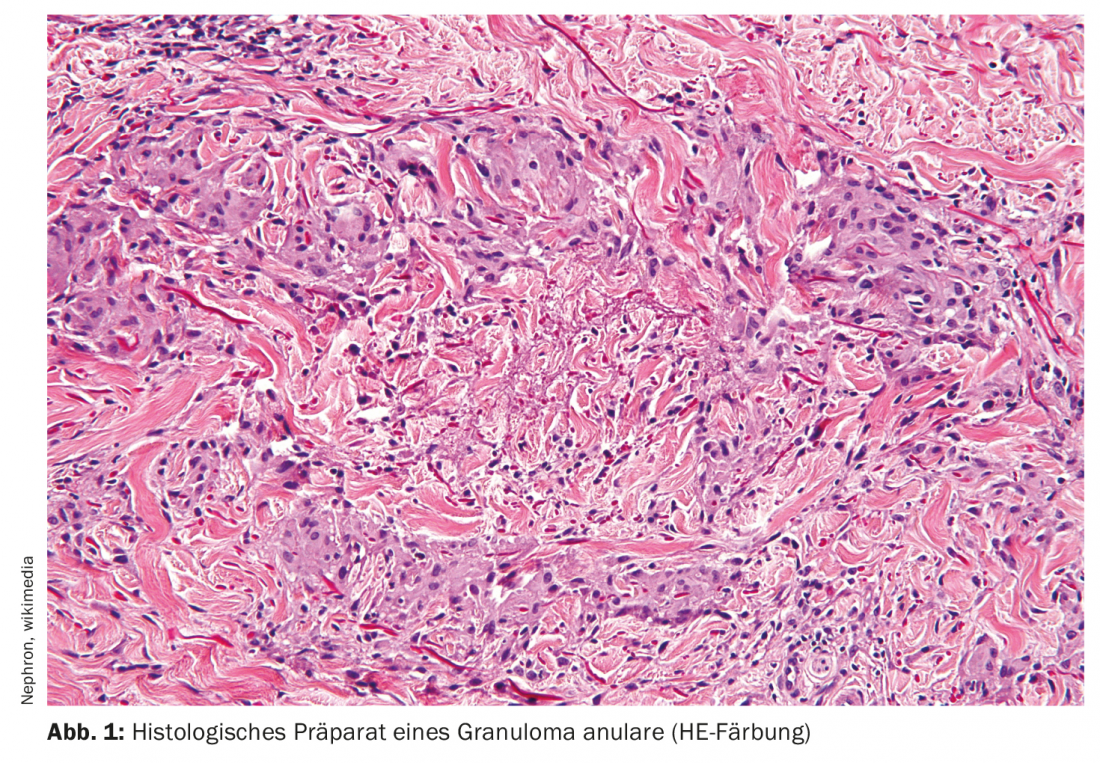
In granuloma anulare (Fig. 1) , locally applied steroids are also the drugs of first choice [5]. Preferably applied topically in children, also intralesionally in adults. A variety of therapies have been described for disseminated forms. In addition to phototherapy (PUVA, UVA-1), treatment with systemic steroids, fumaric acid esters, dapsone, or hydroxychloroquine may be considered [5].


Evidence of efficacy for the use of the JAK inhibitor tofacitinib is also found in the literature, both in sarcoidosis (review 1) and granuloma anulare (review 2) [6,7]. Tofacitinib is a JAK inhibitor, which is also used in other skin diseases. Janus kinases (JAK) play a role in the phosphorylation of STAT molecules and are involved in the signal transduction of many different cytokines. The anti-inflammatory effect is based on the blockade of Janus kinases, on which cytokine receptors depend to exert their effect as inflammatory mediators via an intracellular signaling cascade. Further studies will show whether the previous findings are confirmed and which patients may benefit most from treatment with JAK inhibitors.
Congress: DDG Conference 2021
Literature:
- Fabri M: Pathomechanisms and therapeutic approaches in granulomatous diseases. Prof. Mario Fabri, MD, PV03: Plenary Lectures 3, DDG Meeting 2021, 04/16/2021.
- Mempel M: Granulomatous diseases. Brown-Falcos Dermatology, Venereology and Allergology. www.springermedizin.de/emedpedia/braun-falcos-dermatologie-venerologie-und-allergologie (last accessed 19.05.2021)
- Inkeles MS, et al: Cell-type deconvolution with immune pathways identifies gene networks of host defense and immunopathology in leprosy. JCI Insight 2016 Sep 22; 1(15): e88843.
- Graf L, Geiser T: The chameleon among systemic diseases: “Sarcoidosis,” Swiss Med Forum 2018; 18(35): 695-701.
- Mempel M: Noninfectious Granulomas of the Skin, 24. October 2017, https://derma.plus/journal/nicht-infektioese-granulome-der-haut (last accessed 19 May 2021).
- Damsky W, et al: Tofacitinib Treatment and Molecular Analysis of Cutaneous Sarcoidosis. N Engl J Med 2018; 379: 2540-2546.
- Wang A, et al: Treatment of granuloma annulare and suppression of proinflammatory cytokine activity with tofacitinib. J Allergy Clin Immunol 2021; 147(5): 1795-1809.
DERMATOLOGIE PRAXIS 2021; 31(3): 36-37 (published 5/31/21, ahead of print).