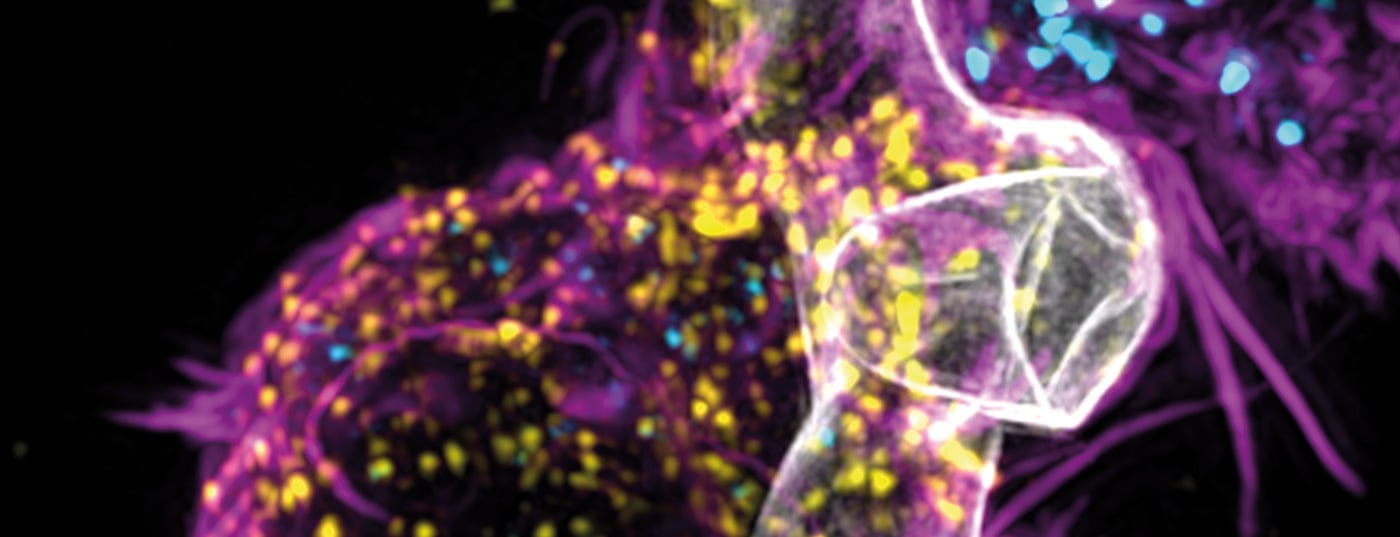
Aspergillus fumigatus is a common mold that is relatively harmless if you have a good immune response. However, in people whose immune system is severely weakened – for example, after chemotherapy or bone marrow transplants – it can cause serious, even fatal infections. Aspergillus fumigatus can also affect lung function in association with COVID or influenza infection.
The infections typically manifest as invasive pulmonary aspergillosis (IPA). Adaptive and innate immune cells responsive to A. fumigatus responsive, are present in the endogenous repertoire of patients with IPA but are rare and cannot be consistently isolated and expanded for adaptive immunotherapy. Researchers from Würzburg have therefore genetically engineered A. fumigatus-specific chimeric antigen receptor (Af-CAR) T cells and demonstrated their ability to confer antifungal reactivity in preclinical models in vitro and in vivo [1].
Through genetic modification, the body’s immune defenses against this type of mold are better activated and more effectively protected. Chimeric antigen receptors (CAR) enable T cells to better recognize the specific structure of the mold and destroy it by releasing certain endogenous messengers.
The development and clinical implementation of CAR-T cell therapies has been established in tumor diseases for years. The patients’ immune cells are reprogrammed to specifically equip them to destroy cancer cells. The chimeric antigen receptor helps T cells recognize and eliminate cancer cells. However, the T cells with the Aspergillus fumigatus-specific CARs (Af-CARs) not only act directly against the fungus, but also indirectly. Thus, in preclinical laboratory models, it has been observed that Af-CAR T cells are able to reach the site of fungal infection and control the recruitment and activation of additional cells of the body’s immune system. Specifically, Af-CAR T cells activate macrophages, enhancing the immune system’s action against mold, the authors write.

Greater antifungal efficacy
Scientists generated a CAR targeting domain AB90-E8 that recognizes a conserved protein antigen in the cell wall of A. fumigatus hyphae. T cells expressing Af-CAR recognized A. fumigatus strains and clinical isolates and exerted direct antifungal activity against A. fumigatus hyphae.
Specifically, CD8+ Af-CAR-T cells released perforin and granzyme B and damaged A. fumigatus hyphae. CD8+ and CD4+ Af-CAR-T cells produced cytokines that activated macrophages to enhance antifungal activity. In an in vivo model of IPA in immunocompromised mice, CD8+ Af-CAR-T cells activated innate immune cells and reduced fungal burden in the lung. Adaptive transfer of CD8+ Af-CAR-T cells resulted in greater antifungal efficacy compared with CD4+ Af-CAR-T cells and improved overall survival.
Overall, the study illustrates the potential of genetically engineered T cells to treat aggressive infectious diseases that are difficult to control with conventional antimicrobial therapy and supports the clinical development of Af-CAR T cell therapy for the treatment of IPA- The Würzburg researchers now plan to implement and evaluate Af-CAR T cell therapy for the treatment of Aspergillus fumigatus infections in initial clinical trials.
Literature:
- CAR T cells targeting Aspergillus fumigatus are effective at treating invasive pulmonary aspergillosis in preclinical models. Journal Science Translational Medicine 2022; doi: 10.1126/scitranslmed.abh1209.
InFo PNEUMOLOGY & ALLERGOLOGY 2022, 4(4): 25.