Small airways disease (SAD) is a central feature of chronic obstructive pulmonary disease (COPD). More than half of all asthma patients also suffer from it. A team of authors analyzed recent studies on the relationship between SAD and emphysema.
By definition, the small and smallest airways are <2 mm in diameter and arise from the 4th-13th generation of airway branching, with the trachea as the 1st generation extending to the alveolus as the 23rd generation. However, normally they occur only up to the 8th generation. Estimates suggest that about 20% of small airways less than 2 mm in diameter have bronchi with cartilaginous elements in their walls, the remainder being bronchioles or ductus alveolaris.
Relationship between SAD and emphysema
Centrilobular emphysema is strongly associated with chronic cigarette smoking. It attacks the secondary lung lobules, which are irregular, polyhedral units of the lung structure (Fig. 1). Secondary lung lobules contain 3-10 acini, as Dr. Andrew Higham, University of Manchester, and his colleagues write in their review paper [1]. Each acinus includes the respiratory bronchioles, alveolar ducts, and alveolar spaces. The emphysematous destruction, which typically results from smoking, originates from the center of the lobule. The respiratory bronchioles, which are mainly affected by centrilobular emphysema, are located in the center. In contrast, panlobular emphysema attacks all structures, from the distal alveoli to the bronchioles of the airways. Paraseptal emphysema involves the alveoli and ducti alveolaris, while sparing the proximal structures.
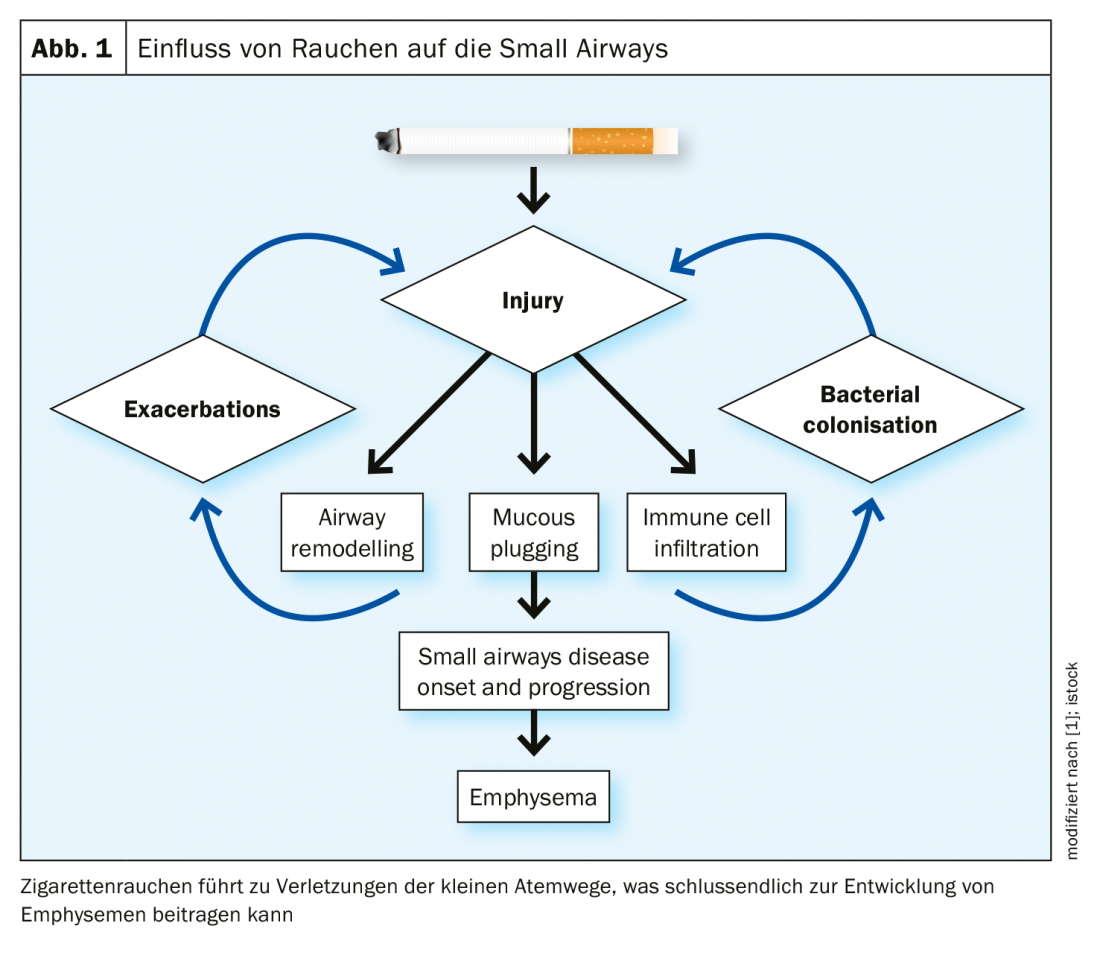
How is small airway remodeling, particularly fibrosis, related to emphysema? On the one hand, there is a thickening of the tissue, but on the other hand, there is a loss of tissue. As Higham et al. a study [2] investigated the expression of 54 tissue repair genes in the small airways and surrounding parenchyma using laser capture microdissections. What was found was differential expression of these genes between the two areas, with expression favoring the parenchymal degradation that surrounds the small airways. The study authors suggest that some small airways are similarly destroyed as the parenchyma, while others have a thickened profile.
Studies support connection
Several recent studies have demonstrated a reduction in the total number of terminal and first order respiratory (transitional) bronchioles in COPD patients compared to controls using microcomputed tomography. 90% of terminal bronchioles were obliterated in stage IV COPD lungs. In GOLD 1 patients, the reduction in terminal and respiratory bronchioles was 29 and 41%, respectively, and in GOLD 2 patients, 40 and 53%, respectively. The remaining small airways had thickened walls and narrowed lumens caused by mucoid obstruction and collagenous debris. The loss and remodeling of terminal and transient bronchioles in lung tissue not affected by emphysema provides further evidence that SAD precedes emphysematous lesions.
SAD, Dr. Higham and his colleagues said, is present in all stages of COPD. The fact that it is also of great importance in the early stages of the disease is now increasingly coming into focus. New research and the resulting findings provide insights into the progression of SAD as well as the importance of exacerbations in promoting inflammatory and remodeling processes associated with small airways disease. SAD appears to be a precursor to the development of emphysema, and therapeutic strategies targeting the small airways in COPD may reduce the rate of emphysema progression. Based on this, the researchers advise that pharmacologic targeting should be applied earlier rather than later in the development of COPD.
Literature:
- Higham A, et al: The pathology of small airways disease in COPD: historical aspects and future directions. Respiratory Research 2019; 20: 49
(https://doi.org/10.1186/s12931-019-1017-y) - Gosselink JV, Hayashi S, Elliott WM, et al: Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 181(12): 1329-1335.
InFo PNEUMOLOGY & ALLERGOLOGY 2019; 1(2): 28.